Abstract
Background:
Although the prevalence of obesity is higher among women than men, they are somewhat protected from the associated cardiometabolic consequences. The increase in cardiovascular disease risk seen after the menopause suggests a role for estrogens. There is also growing evidence for the importance of estrogen on body fat and metabolism in males. We hypothesized that that estrogen administration would ameliorate the adverse effects of obesity on metabolic parameters in males.
Methods:
Male and female C57Bl/6 mice were fed control or obesogenic (DIO) diets from 5 weeks of age until adulthood. Glucose tolerance testing was performed at 13 weeks of age. Mice were killed at 15 weeks of age and liver and adipose tissue were collected for analysis of gene expression. A second cohort of male mice underwent the same experimental design with the addition of estradiol pellet implantation or sham surgery at 6 weeks.
Results:
DIO males had greater mesenteric adipose deposition and more severe increases in plasma glucose, insulin and lipids than females. Treatment of males with estradiol from 6 weeks of age prevented DIO-induced increases in adipose tissue mass and alterations in glucose–insulin homeostasis. We also identified sex differences in the transcript levels and activity of hepatic and adipose glucocorticoid metabolizing enzymes. Estrogen treatment feminized the pattern of DIO-induced changes in glucocorticoid metabolism, rendering males similar to females.
Conclusions:
Thus, DIO induces sex-specific changes in glucose–insulin homeostasis, which are ameliorated in males treated with estrogen, highlighting the importance of sex steroids in metabolism. Given that altered peripheral glucocorticoid metabolism has been observed in rodent and human obesity, our results also suggest that sexually dimorphic expression and activity of glucocorticoid metabolizing enzymes may have a role in the differential metabolic responses to obesity in males and females.
Similar content being viewed by others
Introduction
There has been a rapid increase in the worldwide prevalence of obesity over recent decades, with a parallel increase in obesity-associated cardiometabolic disorders.1 Although the prevalence of obesity is higher among women, they may be somewhat protected from the associated cardiometabolic consequences, at least until the menopause.2 This is also the case in rodents: male mice are more prone to diet-induced obesity3, 4 and metabolic dysfunction5, 6 than females and there are sex differences in the response to a high-fat diet in genetic models of obesity and diabetes in rats.7, 8 A role for sex steroids, particularly estrogens, in modulating glucose and insulin homeostasis is supported by observations in humans: pre-menopausal women have higher insulin sensitivity when compared with age-matched men;9, 10 and following the menopause, women tend to accumulate visceral fat and become more insulin resistant, with a consequent increase in the risk of type 2 diabetes.11 An increasing body of evidence suggests that estrogens also have important beneficial effects on body fat and metabolism in males.12, 13, 14, 15 We used a mouse model of diet-induced obesity to investigate sex differences in susceptibility to the metabolic consequences of obesity by addressing the hypothesis that estrogen treatment in males would ameliorate the adverse effects of diet-induced obesity on metabolic parameters. Given that altered peripheral glucocorticoid metabolism has been observed in rodent and human obesity, we also sought to explore the potential role of sex differences in glucocorticoid metabolism.
Materials and methods
Animals and experimental design
All animal procedures were carried out under UK Home Office license approval under the Animals (Scientific Procedures) Act, 1986, and with local ethical committee approval. C57Bl/6 mice were bred in-house and maintained under controlled conditions of light (on 0700–1900 hours) and temperature (21 °C) with free access to food and water. Males and females (n=8 per group) were weaned aged 3 weeks onto standard chow (Special Diet Services Witham, Essex, UK) and at 5 weeks were randomly assigned by a technician not involved in the research to high-fat, high-sugar ‘obesogenic’ diet (DIO: D12328, Research Diets, New Brunswick, NJ, USA) or control diet (CON: D12331, Research Diets). D12328 induces obesity in rodents16 and the corresponding control diet was matched in terms of protein and micronutrient content. Animals were weighed weekly and after 8 weeks were individually housed to allow for metabolic investigation. Animals were killed aged 15 weeks by CO2 asphyxiation, between 1400 and 1600 hours following a 6-h fast, and trunk blood was collected. Tissues were dissected immediately, weighed and snap frozen on dry ice. Females were killed during estrus, confirmed by vaginal smear examination showing exclusively non-nucleated cornified epithelial cells.
In a separate experiment, pre-pubertal C57Bl/6 mice of both sexes were killed following weaning (3–4 weeks of age; n=7 per group), after a physical examination to exclude puberty (vaginal opening in females and balanopreputial separation in males). Tissues were dissected immediately, weighed and snap frozen on dry ice.
A second cohort of male mice underwent the same experimental design with the addition of estradiol pellet implantation or sham surgery at 6 weeks (1 week post initiation of experimental diet; n=11 per sham group, 12 per estradiol group). Animals were anaesthetized using isoflurane and 90-day, 0.25 mg 17β-estradiol pellets (Innovative Research of America, Sarasota, FL, USA) implanted subcutaneously in the left mid-dorsal region. Skin incisions were closed using silk sutures. Sham-operated animals underwent the same surgical procedure with the exclusion of the pellet being inserted. Animals were killed aged 15 weeks using the same protocol as listed in experimental design. Plasma concentrations of 17β-estradiol were determined using an enzyme-linked immunosorbent assay (Calbiotech, Spring Valley, CA, USA) on blood samples taken when animals were killed.
Metabolic measures
Glucose tolerance tests were performed at 13 weeks of age; animals were fasted for 6 h and tests commenced at 1400 hours. A basal blood sample was taken by tail nick followed by an intraperitoneal injection of a glucose load (2 g kg−1) and blood samples collected 15, 30, 60 and 90 min post injection. Samples were centrifuged at 2.3 g for 10 min and plasma stored at −20 °C. Plasma glucose, cholesterol and triglyceride concentrations were measured by enzymatic methods (ThermoElectron, Pittsburgh, PA, USA). Insulin (Crystal Chem Inc., Chicago, IL, USA) and testosterone (Demeditec Diagnostics GmbH, Kiel, Germany) were determined by enzyme-linked immunosorbent assay. Non-esterified fatty acid concentrations were determined using a kit (Wako Diagnostics, Richmond, VA, USA). At 14 weeks of age, plasma corticosterone was measured in tail tip blood samples taken at 0700 and 1900 within 1 min of cage disturbance, using an in-house radio-immuno assay.17 Hepatic triglyceride was extracted by saponification18 and quantified as in plasma.
Quantification of mRNA
Total RNA was extracted from snap frozen tissues using an RNeasy mini kit (Qiagen, West Sussex, UK) and reverse transcribed (250 ng liver and 500 ng adipose) using the Access RT-PCR kit from Promega (Hampshire, UK). Real-time PCR was performed using a Roche Lightcycler 480 (Roche Diagnostics Ltd., Sussex, UK) with Universal Probes master mix (Roche) and thermal cycler parameters recommended by the manufacturer. PCR was performed in triplicate in a total volume of 10 μl containing 2 μl of complementary DNA, 5 μl Roche master mix, 12 pmol μl−1 primers and 4 pmol μl−1 probe (Universal probe library, Roche); primer sequences and probe numbers are given in Table 1. A standard curve was constructed for each primer/probe set using a serial dilution of complementary DNA pooled from all samples. Expression was corrected for cyclophilin A (Taqman gene expression assay Applied Biosytems, Warrington, UK; Mm02342430_g1) in the liver and beta-actin (Mm01215647_g1) in adipose tissue.
11β-HSD1 activity assay
11β-HSD1 is a reductase in vivo, converting inactive 11-dehydrocorticosterone to corticosterone. However, in vitro, dehydrogenase activity predominates and measurements of reductase activity are confounded by competition with other enzymes. Therefore, as an index of total 11β-HSD1 protein, we measured enzyme activity as conversion of corticosterone to 11-dehydrocorticosterone in the presence of an excess of the cofactor NADP+ as previously described.19 Reactions were optimized to ensure first-order kinetics (liver 0.025 mg ml−1 for 2–4 h; adipose 0.025–0.1 mg ml−1 for 16–24 h).
Statistical analysis
Data presented are mean±s.e.m. Numbers were based on our previous studies.20 Statistical analysis was carried out by t-testing, two-way or repeated measures analysis of variance as appropriate. Data were normally distributed and groups compared had similar variances. Values were considered different when P<0.05.
Results
Effects of DIO on glucose homeostasis, lipids and plasma corticosterone in males and females
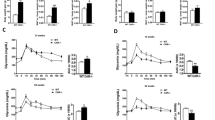
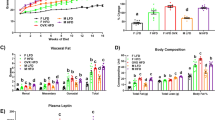
Consumption of an obesogenic diet caused a significant increase in body weight in males (Figure 1a) and an increase in adipose tissue weights in both the sexes (Figure 1b). Plasma triglyceride concentrations were lower in CON females compared with males (Table 2) but plasma cholesterol and plasma and hepatic triglyceride concentrations were similarly increased by DIO in both the sexes (Table 2). DIO increased plasma concentrations of glucose (Figure 1c) and insulin (Figure 1d) following a glucose load, with a substantially larger effect in males. In mice on control diet, nadir plasma corticosterone concentrations were comparable in both the sexes; however, peak concentrations were higher in females than in males (Table 2). Peak plasma corticosterone was reduced by DIO in both the sexes (Table 2).
Effect of obesogenic diet and sex on adiposity and metabolism in C57Bl/6 mice. Male and female mice were fed control (CON) or obesogenic diet (DIO) from 5 weeks of age. (a) Body weight during the study, (b) adipose mass as percentage of body weight at killing. SC, subcutaneous; Mes, mesenteric; RP, retroperitoneal; Epi, epididymal. Plasma glucose (c) and insulin (d) during a glucose tolerance test. Area under curve (AUC) glucose: male CON 1003.2±97.5; male DIO 1285.2±93.7; female CON 859.1±65.8; female DIO 961.5±126.3. AUC insulin: male CON 38.9±6.9; male DIO 160.0±22.3; female CON 28.9±1.9; female DIO 55.7±7.4. Data are mean±s.e.m., n=8 per group, analyzed by repeated measures analysis of variance (ANOVA) (a, c and d) and two-way ANOVA (b), repeated measures where necessary. Effect of diet **P<0.01, ***P<0.001; effect of sex †P<0.05, ††P<0.01, †††P<0.001.
Effects of estradiol administration in male mice
At the end of the experiment, plasma estradiol concentrations were increased in males treated with estradiol compared with the sham group, and were significantly higher in the estradiol-treated DIO group compared with all other groups (Table 3). Plasma testosterone concentrations were reduced by estradiol treatment but unaffected by diet (Table 3). Estradiol administration reduced weight gain (Figure 2a) and adipose tissue weight (Figure 2b) in both CON and DIO males. Estradiol also ameliorated the increase in plasma glucose and insulin caused by DIO (Figures 2c and d) but did not affect plasma triglyceride or cholesterol concentrations (Table 3). However, estrogen treatment reversed the DIO-associated increase in hepatic triglyceride (Table 3). Nadir plasma concentrations of corticosterone were higher in estradiol-treated males but were unaffected by DIO; peak concentrations were comparable in all the groups (Table 3).
Effect of obesogenic diet and estrogen treatment on adiposity and metabolism in male mice. Male mice were fed control (CON) or obesogenic (DIO) diet from 5 weeks of age and at 6 weeks underwent implantation of a continuous release 17β-estradiol pellet (estradiol) or sham surgery (sham). (a) Body weight over duration of study. (b) Adipose mass as percentage of body weight. SC, subcutaneous; Mes, mesenteric; RP, retroperitoneal; Epi, epididymal. Plasma glucose (c) and insulin (d) during a glucose tolerance test. AUC glucose: sham CON 859.2±123.7; sham DIO 1290.6±110.8; estrodiol CON 596.9±32.3; estrodiol DIO 759.3±46.3. AUC insulin: sham CON 41.0±5.8; sham DIO 185.8±9.9; estrodiol CON 44.8±10.7; estrodiol DIO 55.0±6.5. Data are mean±s.e.m., n=11 per sham group, 12 per estradiol group, analyzed by repeated measures analysis of variance (ANOVA) (a, c and d) or two-way ANOVA (b), repeated measures where necessary. Effect of diet ***P<0.001; effect of estradiol †††P<0.001; interaction between effects ∫P<0.05, ∫∫P<0.01, ∫∫∫P<0.001.
Glucocorticoid metabolism in DIO and the effects of estrogen
Given the sex differences in circulating corticosterone levels and the fact that altered glucocorticoid metabolism may contribute to the pathogenesis of obesity and related metabolic disorders,21 we proceeded to measure transcript and expression levels of glucocorticoid metabolizing enzymes including 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1, which reactivates glucocorticoids from their inert 11-keto metabolites) and the levels of the A-ring reductases (5α- and 5β-reductase, which convert glucocorticoids into their dihydro metabolites). Females had lower hepatic transcript levels of 5α-reductase and higher transcript levels of 5β-reductase than males (Figure 3a). There was a trend for females to have higher hepatic transcript levels of 11β-HSD1 than males (P=0.057; Figure 3a) but there were no differences in hepatic enzyme activity (Figure 3b). In subcutaneous fat, although there were no sex differences in transcript levels of 11β-HSD1 (Figure 3c), enzyme activity was lower in females compared with males (Figure 3d). DIO increased hepatic transcript levels of 11β-HSD1 in males but not in females (Figure 3a), although there were no changes in hepatic enzyme activity (Figure 3b). There was no effect of DIO on hepatic transcript levels of 5α-reductase or 5β-reductase in either sex (Figure 3a). In subcutaneous adipose tissue, DIO reduced 11β-HSD1 expression (Figure 3c) and activity (Figure 3d) in both the sexes.
Effect of obesogenic diet and sex on glucocorticoid metabolism. Male and female mice were fed control (CON) or obesogenic diet (DIO) from 5 weeks of age. mRNA abundance of glucocorticoid metabolizing genes was assessed using real-time PCR in the liver (a) and subcutaneous adipose (c). Activity of 11β-HSD1 was analyzed in samples from the same tissues (b and d). Data are mean±s.e.m., n=8 per group, analyzed by two-way analysis of variance. Effect of diet ***P<0.001; effect of sex †††P<0.001; interaction between diet and sex ∫∫P<0.01.
To investigate when the sex differences in glucocorticoid metabolizing enzymes became apparent, a cohort of mice were killed before puberty (when sex-steroid concentrations are low) and enzyme expression and activity were determined. In pre-pubertal mice, there was a trend for a lower expression of hepatic 5α-reductase in females (Figure 4a), but no differences in the expression of hepatic 5β-reductase or in the expression or activity of 11β-HSD1 (Figures 4a and b). Furthermore, expression and activity of 11β-HSD1 in adipose tissue were comparable in male and female pre-pubertal mice (Figures 4c and d).
Sex differences in hepatic and subcutaneous fat glucocorticoid metabolism in pre-pubertal mice. Male and female mice were killed aged 3–4 weeks, before signs of puberty, and liver and subcutaneous adipose were collected. mRNA abundance of glucocorticoid metabolizing genes was assessed using real-time PCR in liver (a) and subcutaneous fat (c) and 11β-HSD1 activity determined in liver (b) and subcutaneous fat (d). Data are mean±s.e.m., n=7 per group, analyzed by Student’s t-test.
In adult males, estradiol increased hepatic transcript levels, but not enzyme activity, of 11β-HSD1 (Figures 5a and b). Analysis by two-way analysis of variance revealed an effect of estradiol to reduce hepatic transcript levels of 5α-reductase and increase transcript levels of 5β-reductase in both CON and DIO males (Figure 5a). In adipose tissue, by contrast with the liver, analysis by two-way analysis of variance revealed an overall effect of estradiol to reduce both the expression (Figure 5c) and activity (Figure 5d) of 11β-HSD1 in CON and DIO males. As DIO reduces both expression and activity of 11β-HSD1 in adipose, resulting in lower baseline 11β-HSD1 levels, there was a much smaller relative reduction in both expression and activity in the estradiol-treated DIO group (Figures 5c and d).
Effect of obesogenic diet and estrogen treatment on glucocorticoid metabolism in male mice. Male mice were fed control (CON) or obesogenic (DIO) diet from 5 weeks of age and at 6 weeks underwent implantation of a continuous release 17β-estradiol pellet (estradiol) or sham surgery (sham). mRNA abundance was assessed using real-time PCR in the liver (a) and subcutaneous adipose (c). Activity of 11β-HSD1 was analyzed in samples from the same tissues (b and d). Data are mean±s.e.m., n=11 per sham group, 12 per estradiol group, analyzed by two-way analysis of variance. Effect of diet **P<0.01, ***P<0.001; effect of estradiol ††P<0.01, †††P<0.001; interaction between effects ∫∫∫P<0.001.
Discussion
Our data confirm previous reports showing that male mice are markedly more susceptible than females to the effects of an obesogenic diet.3, 4, 5, 6 In postmenopausal women and in female animal models, lower estrogen levels are associated with increased visceral adiposity22, 23 and estrogen replacement improves glucose–insulin homeostasis.24, 25 We show that estrogen treatment of males is associated with an improvement in weight gain and DIO-induced metabolic changes, supporting the concept that estrogen has an important role in the control of metabolism and adiposity. There is growing evidence for a fundamental role for estrogen in the regulation of obesity and related metabolic disorders in males12, 13, 14 and recent data from rodent studies suggest that hepatic estrogen signaling has a key role in the prevention of high-fat diet-induced insulin resistance in males.26 Indeed, the aromatization of testosterone to estradiol may underpin many of the physiological effects which have generally been attributed to the action of testosterone, for example, the prevention of visceral adiposity.12 Estrogen may also have a role in the control of hepatic lipid metabolism and hepatic lipid deposition,26, 27, 28 and estrogen treatment in DIO males abolished the DIO-induced increase in the accumulation of hepatic triglyceride. Intriguingly, this occurred in the absence of any effect of estradiol on plasma triglyceride and cholesterol concentrations. Estrogen may influence appetite and energy expenditure.29 Although we did not measure food intake and energy expenditure in this study, data from female rodents suggest that ovariectomy induces an increase in food intake and estrogen replacement decreases food intake.29 However, hyperphagia does not fully account for the changes in metabolism and development of obesity after ovariectomy.29 Our findings, showing the importance of estrogen signaling in regulating body weight, glucose–insulin homeostasis and hepatic triglyceride content are in agreement with studies in mice lacking the estrogen receptor (ER). Mice lacking ER have increased adipose tissue, higher fasting blood glucose and insulin30 and hepatic insulin resistance with altered hepatic lipid handling.26, 31 Furthermore, deletion of ERα in mice blocks the antiobesity effects of estrogen replacement.29 Male mice lacking ER specifically in the liver show reduced insulin sensitivity.26 Conversely, hepatic ERα overexpression is associated with markedly reduced hepatic triglyceride content and improved insulin sensitivity.32
There were sex differences in circulating corticosterone concentrations and, as in previous rodent studies, DIO reduced peak corticosterone concentrations in both the sexes,33, 34 which may reflect altered peripheral glucocorticoid clearance.35, 36 As altered glucocorticoid metabolism may contribute to the pathogenesis of obesity and related metabolic disorders,21 we hypothesized that some of the protective effects of estrogen might be due to effects on adipose and/or hepatic glucocorticoid metabolism. There are sex differences in the expression and activity of glucocorticoid metabolizing enzymes in humans, with lower expression and activity of hepatic and adipose 11β-HSD1 in females37 and estrogen regulates 11β-HSD1 expression and activity in the rat liver38 and kidney,39 and in rodent and human adipocytes.40, 41 We observed sex-specific responses of glucocorticoid metabolizing enzymes to diet-induced obesity, and estrogen therapy feminized the pattern of enzyme expression and activity in males. We observed no sex differences in the expression or activity of hepatic or adipose 11β-HSD1 in pre-pubertal mice, although there were clear sex differences in adult animals. 11β-HSD1 messenger RNA (mRNA) and activity were higher in lean males compared with females, a finding which has also been reported in humans.37 This predicts greater regeneration of corticosterone in adult male adipose tissue and may be a disadvantage given that mice overexpressing 11β-HSD1 in adipose tissue exhibit intra-abdominal obesity and metabolic dysfunction.42 The DIO-induced reduction in 11β-HSD1 mRNA and activity in subcutaneous adipose tissue that occurred in both the sexes in our study has previously been reported in male rodents34, 43 and has been proposed as a protective mechanism to reduce both circulating glucocorticoid concentrations and local glucocorticoid signaling.34, 43 Indeed, 11β-HSD1-knockout mice, or mice treated with selective 11β-HSD1 inhibitors, are resistant to obesity and hyperglycemia when fed a high-fat diet,44, 45, 46, 47, 48 although, notably, these studies were carried out in male animals. Lower adipose 11β-HSD1 in females compared with males may, therefore, contribute to the relative protection of females from the metabolic effects of obesity. Consistent with this hypothesis, the estrogen-induced reduction in adipose 11β-HSD1 mRNA and activity may be one mechanism for the protection from the metabolic consequences of high-fat diet in estrogen-treated males. However, the relative importance of changes in adipose tissue 11β-HSD1 in mediating the metabolic response to DIO remains unclear, as DIO itself is associated with a profound reduction in the expression and activity of adipose 11β-HSD1 in both the sexes, yet the metabolic phenotype is more severe in DIO males.
Sex differences in the expression of 5α- and 5β-reductase also became apparent after puberty. As 5α-reduced glucocorticoid metabolites are active at the glucocorticoid receptor,49 the increased expression of 5α-reductase and lower expression of 5β-reductase in males predict increased hepatic concentrations of active glucocorticoids in males and may render them more susceptible to the effects of a high-fat diet. Indeed, in humans obesity is associated with alterations in the ratio of urinary 5α- and 5β-reduced glucocorticoid metabolites, with an increased proportion of cortisol metabolized by 5α-reduction.21, 36, 50 Estradiol treatment in males decreased hepatic 5α-reductase and increased 5β-reductase expression, resulting in a similar expression pattern to that in females. As 5β-reductase can metabolize both corticosterone and 11-dehydrocorticosterone, and its metabolites are not active at glucocorticoid receptor, this predicts reduced intrahepatic glucocorticoid signaling and may be one mechanism by which estrogen treatment resulted in protection from the metabolic consequences of exposure to a high-fat diet. Although females, DIO males and estradiol-treated males had increased hepatic 11β-HSD1 expression in comparison to lean males, this was not reflected in differences in enzyme activity. We have previously suggested that this discrepancy may reflect post-transcriptional modification.34 Intriguingly, the DIO-induced increase in hepatic 11β-HSD1 and the reduction in peak corticosterone were not present in the sham surgery DIO males suggesting that there may be long-term effects of surgery on glucocorticoid metabolism.
In terms of mechanisms, changes in 11β-HSD1 expression following ovariectomy and estrogen treatment in female rats could be due to reduced adiposity rather than increases in estradiol.51 However, both direct and indirect effects of estradiol on adipose 11β-HSD1 have been proposed: estradiol is a competitive inhibitor of 11β-HSD1 in primary cultures of rat adipocytes40 and higher adipose expression of 11β-HSD1 is found in postmenopausal women.41 In our study, the lack of sex difference in adipose and liver 11β-HSD1 expression and activity in pre-pubertal mice, when sex-steroid concentrations are low, combined with the changes with estrogen treatment in adult males are consistent with direct regulation of glucocorticoid metabolism in adipose tissue. Although estrogen may have an important influence on peripheral glucocorticoid metabolism, there is increasing evidence for the key role of insulin.52, 53, 54, 55 In rodents, insulin senitization ameliorates the obesity-induced changes in hepatic A-ring reductase expression and activity,55 and in humans, intravenous insulin acutely increases cortisol regeneration by 11β-HSD1(ref. 54) and cortisol production by 11β-HSD1 parallels the change in circulating insulin concentrations following meals.52 Thus, the changes in hepatic and adipose glucocorticoid metabolism seen in females and in estrogen-treated males may additionally represent a downstream effect of the marked improvement in insulin sensitivity. Finally, the changes induced by estrogen treatment may also be attributed to reduced testosterone levels in both CON and DIO males. However, a recent study in humans in which endogenous testosterone and estradiol were suppressed pharmacologically suggests that, whereas androgen deficiency may account for decreases in lean mass, estrogen deficiency is responsible for increases in body fat.12 Nevertheless, altered estrogen/androgen balance may still be of importance in the maintenance of normal physiology and future studies in which testosterone levels are maintained at physiological levels would help to determine the relative importance of estrogens versus androgens.
Our hypothesis for this study was that estrogen treatment in males would ameliorate the adverse effects of diet-induced obesity on metabolic parameters. Consequently, we did not assess the effects of administration of estradiol in females. Estrogens may also have a role in mediating glucose–insulin homeostasis in women and estrogen deficiency is associated with an increasing risk of obesity, the metabolic syndrome and type 2 diabetes.29 In postmenopausal women, the administration of estrogen can improve glucose homeostasis, insulin sensitivity and lipid profile.56, 57, 58 Studies using estrogen replacement in ovariectomized mice have shown that estrogen protects against fatty liver and may improve pathway-selective insulin resistance.28
In conclusion, our data support the importance of estrogen in the apparent protection of females from the deleterious effects of exposure to an obesogenic diet and provide further evidence for the suggestion that manipulating estrogen signaling pathways may represent an alternative/additional approach to the management of the complications of obesity in males. In addition, they suggest that sexually dimorphic expression and activity of glucocorticoid metabolizing enzymes may contribute to gender differences in metabolic responses to diet-induced obesity. Understanding the molecular basis of sex differences in disease risk may provide new approaches to the management of obesity-associated metabolic disease.
References
Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C et al. Waist circumference and cardiometabolic risk: a consensus statement from Shaping America's Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Am J Clin Nutr 2007; 85: 1197–1202.
Kautzky-Willer A, Handisurya A . Metabolic diseases and associated complications: sex and gender matter!. Eur J Clin Invt 2009; 39: 631–648.
Noonan WT, Banks RO . Renal function and glucose transport in male and female mice with diet-induced type II diabetes mellitus. Proc Soc Exp Biol Med 2000; 225: 221–223.
Nishikawa S, Yasoshima A, Doi K, Nakayama H, Uetsuka K . Involvement of sex, strain and age factors in high fat diet-induced obesity in C57BL/6J and BALB/cA mice. Exp Anim 2007; 56: 263–272.
Hwang LL, Wang CH, Li TL, Chang SD, Lin LC, Chen CP et al. Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity 2010; 18: 463–469.
Stubbins R, Holcomb V, Hong J, Núñez N . Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutrition 2012; 51: 861–870.
Maher MA, Banz WJ, Truett GE, Zemel MB . Dietary fat and sex modify heterozygote effects of the rat Fatty (fa) allele. J Nutr 1996; 126: 2487–2493.
Corsetti JP, Sparks JD, Peterson RG, Smith RL, Sparks CE . Effect of dietary fat on the development of non-insulin dependent diabetes mellitus in obese Zucker diabetic fatty male and female rats. Atherosclerosis 2000; 148: 231–241.
Donahue RP, Bean JA, Donahue RA, Goldverg RB, Prineas RJ . Insulin response in a triethnic population: effects of sex, ethnic origin, and body fat. Miami Community Health Study. Diabetes Care 1997; 20: 1670–1676.
Nuutila P, Knuuti MJ, Mäki M, Laine H, Ruotsalainen U, Teräs M et al. Gender and insulin sensitivity in the heart and in skeletal muscles. Studies using positron emission tomography. Diabetes 1995; 44: 31–36.
Carr MC . The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 2003; 88: 2404–2411.
Finkelstein JS, Lee H, Burnett-Bowie S-AM, Pallais JC, Yu EW, Borges LF et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med 2013; 369: 1011–1022.
Fox CS, Yang Q, Cupples LA, Guo C-Y, Atwood LD, Murabito JM et al. Sex-specific association between estrogen receptor-α gene variation and measures of adiposity: The Framingham Heart Study. J Clin Endocrinol Metab 2005; 90: 6257–6262.
Maffei L, Murata Y, Rochira V, Tubert G, Aranda C, Vazquez M et al. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. J Clin Endocrinol Metabol 2004; 89: 61–70.
Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 1994; 331: 1056–1061.
Surwit RS, Wang S, Petro AE, Sanchis D, Raimbault S, Ricquier D et al. Diet-induced changes in uncoupling proteins in obesity-prone and obesity-resistant strains of mice. Proc Natl Acad Sci USA 1998; 95: 4061–4065.
Holmes MC, French KL, Seckl JR . Modulation of serotonin and corticosteroid receptor gene expression in the rat hippocampus with circadian rhythm and stress. Brain Res Mol Res 1995; 28: 186–192.
Norris AW, Chen L, Fisher SJ, Szanto I, Ristow M, Jozsi AC et al. Muscle-specific PPARgamma-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J Clin Invest 2003; 112: 608–618.
Livingstone DE, Jones GC, Smith K, Jamieson PM, Andrew R, Kenyon CJ et al. Understanding the role of glucocorticoids in obesity: tissue-specific alterations of corticosterone metabolism in obese Zucker rats. Endocrinology 2000; 141: 560–563.
King V, Dakin RS, Liu L, Hadoke PW, Walker BR, Seckl JR et al. Maternal obesity has little effect on the immediate offspring but impacts on the next generation. Endocrinology 2013; 154: 2514–2524.
Rask E, Olsson T, Soderberg S, Andrew R, Livingstone DE, Johnson O et al. Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab 2001; 86: 1418–1421.
Kotani K, Tiokunaga K, Fujioka S, Kobatake T, Keno Y, Yoshida S et al. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. Int J Obes Relat Metab Dis 1994; 18: 207–212.
D'Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS . Estrogen regulation of adiposity and fuel partitioning: evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem 2005; 280: 35983–35991.
Riant E, Waget A, Cogo H, Arnal J-F, Burcelin R, Gourdy P . Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology 2009; 150: 2109–2117.
Nabulsi AA, Folsom AR, White A, Patsch W, Heiss G, Wu KK et al. Association of hormone-replacement therapy with various cardiovascular risk factors in postmenopausal women. N Engl J Med 1993; 328: 1069–1075.
Zhu L, Martinez MN, Emfinger CH, Palmisano BT, Stafford JM . Estrogen signaling prevents diet-induced hepatic insulin resistance in male mice with obesity. Am J Physiol Endocrinol Metab 2014; 306: E1188–E1197.
Camporez JPG, Jornayvaz FR, Lee H-Y, Kanda S, Guigni BA, Kahn M et al. Cellular mechanism by which estradiol protects female ovariectomized mice from high-fat diet-induced hepatic and muscle insulin resistance. Endocrinology 2013; 154: 1021–1028.
Zhu L, Brown WC, Cai Q, Krust A, Chambon P, McGuinness OP et al. Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes 2013; 62: 424–434.
Mauvais-Jarvis F, Clegg DJ, Hevener AL . The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev 2013; 34: 309–338.
Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS . Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci USA 2000; 97: 12729–12734.
Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL et al. Evidence that oestrogen receptor-α plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia 2006; 49: 588–597.
Wang X, Lu Y, Wang E, Zhang Z, Xiong X, Zhang H et al. Hepatic estrogen receptor α improves hepatosteatosis through up-regulation of small heterodimer partner. J Hepatol 2015. e-pub ahead of print 24 February 2015 doi:10.1016/j.jhep.2015.02.029.
Auvinen HE, Romijn JA, Biermasz NR, Pijl H, Havekes LM, Smit JWA et al. The effects of high fat diet on the basal activity of the hypothalamus–pituitary–adrenal axis in mice. J Endocrinol 2012; 214: 191–197.
Drake AJ, Livingstone DEW, Andrew R, Seckl JR, Morton NM, Walker BR . Reduced adipose glucocorticoid reactivation and increased hepatic glucocorticoid clearance as an early adaptation to high-fat feeding in Wistar rats. Endocrinology 2005; 146: 913–919.
Walker BR . Glucocorticoids and cardiovascular disease. Eur J Endocrinol 2007; 157: 545–559.
Rask E, Walker BR, Soderberg S, Livingstone DE, Eliasson M, Johnson O et al. Tissue-specific changes in peripheral cortisol metabolism in obese women: increased adipose 11beta-hydroxysteroid dehydrogenase type 1 activity. J Clin Endocrinol Metab 2002; 87: 3330–3336.
Paulsen SK, Pedersen SB, Fisker S, Richelsen B . 11beta-HSD type 1 expression in human adipose tissue: impact of gender, obesity and fat localisation. Obesity 2007; 15: 1954–1960.
Jamieson PM, Nyirenda MJ, Walker BR, Chapman KE, Seckl JR . Interactions between oestradiol and glucocorticoid regulatory effects on liver-specific glucocorticoid-inducible genes: possible evidence for a role of hepatic 11beta-hydroxysteroid dehydrogenase type 1. J Endocrinol 1999; 160: 103–109.
Gomez-Sanchez EP, Ganjam V, Chen YJ, Liu Y, Zhou MY, Toroslu C et al. Regulation of 11β-hydroxysteroid dehydrogenase enzymes in the rat kidney by estradiol. Am J Physiol 2003; 285: E272–E279.
Tagawa N, Yuda R, Kubota S, Wakabayashi M, Yamaguchi Y, Kiyonaga D et al. 17β-Estradiol inhibits 11β-hydroxysteroid dehydrogenase type 1 activity in rodent adipocytes. J Endocrinol 2009; 202: 131–139.
Andersson T, Simonyte K, Andrew R, Strand M, Burén J, Walker BR et al. Tissue-specific increases in 11β-Hydroxysteroid Dehydrogenase Type 1 in normal weight postmenopausal women. PLoS ONE 2009; 4: e8475.
Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR et al. A transgenic model of visceral obesity and the metabolic syndrome. Science 2001; 294: 2166–2170.
Morton NM, Ramage LE, Seckl JR . Down-regulation of adipose 11beta-hydroxysteroid dehydrogenase type 1 by high-fat feeding in mice: A potential adaptive mechanism counteracting metabolic disease. Endocrinology 2004; 145: 2707–2712.
Kotelevtsev Y, Holmes MC, Burchell A, Houston PM, Schmoll D, Jamieson P et al. 11beta-hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc Natl Acad Sci USA 1997; 94: 14924–14929.
Morton NM, Holmes MC, Fievet C, Staels B, Tailleux A, Mullins JJ et al. Improved lipid and lipoprotein profile, hepatic insulin sensitivity, and glucose tolerance in 11beta-hydroxysteroid dehydrogenase type 1 null mice. J Biol Chem 2001; 276: 41293–41300.
Alberts P, Nilsson C, Selen G, Engblom LO, Edling NH, Norling S et al. Selective inhibition of 11 beta-hydroxysteroid dehydrogenase type 1 improves hepatic insulin sensitivity in hyperglycemic mice strains. Endocrinology 2003; 144: 4755–4762.
Berthiaume M, Laplante M, Festuccia W, Gélinas Y, Poulin S, Lalonde J et al. Depot-specific modulation of rat intraabdominal adipose tissue lipid metabolism by pharmacological inhibition of 11β-hydroxysteroid dehydrogenase type 1. Endocrinology 2007; 148: 2391–2397.
Chapman KE, Kotelevtsev YV, Jamieson PM, Williams LJ, Mullins JJ, Seckl JR . Tissue-specific modulation of glucocorticoid action by the 11 beta-hydroxysteroid dehydrogenases. Biochem Soc Trans 1997; 25: 583–587.
McInnes KJ, Kenyon CJ, Chapman KE, Livingstone DEW, Macdonald LJ, Walker BR et al. 5alpha-reduced glucocorticoids, novel endogenous activators of the glucocorticoid receptor. J Biol Chem 2004; 279: 22908–22912.
Andrew R, Phillips DI, Walker BR . Obesity and gender influence cortisol secretion and metabolism in man. J Clin Endocrinol Metab 1998; 83: 1806–1809.
Paulsen SK, Nielsen MP, Richelsen B, Bruun JM, Flyvbjerg A, Pedersen SB . Upregulation of adipose 11-beta-hydroxysteroid dehydrogenase type 1 expression in ovariectomized rats is due to obesity rather than lack of estrogen. Obesity 2008; 16: 731–735.
Stimson RH, Mohd-Shukri NA, Bolton JL, Andrew R, Reynolds RM, Walker BR . The postprandial rise in plasma cortisol in men is mediated by macronutrient-specific stimulation of adrenal and extra-adrenal cortisol production. J Clin Endocrinol Metab 2014; 99: 160–168.
Stimson RH, Andrew R, McAvoy NC, Tripathi D, Hayes PC, Walker BR . Increased whole-body and sustained liver cortisol regeneration by 11β-hydroxysteroid dehydrogenase type 1 in obese men with type 2 diabetes provides a target for enzyme inhibition. Diabetes 2011; 60: 720–725.
Wake DJ, Homer NZM, Andrew R, Walker BR . Acute in vivo regulation of 11β-hydroxysteroid dehydrogenase type 1 activity by insulin and intralipid infusions in humans. J Clin Endocrinol Metab 2006; 91: 4682–4688.
Livingstone DE, McInnes KJ, Walker BR, Andrew R . Increased A-ring reduction of glucocorticoids in obese Zucker rats: effects of insulin sensitization. Obes Res 2005; 13: 1523–1526.
Andersson B, Mattsson L-Å, Hahn L, MÅrin P, Lapidus L, Holm G et al. Estrogen replacement therapy decreases hyperandrogenicity and improves glucose homeostasis and plasma lipids in postmenopausal women with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1997; 82: 638–643.
Spencer CP, Godsland IF, Cooper AJ, Ross D, Whitehead MI, Stevenson JC . Effects of oral and transdermal 17β-estradiol with cyclical oral norethindrone acetate on insulin sensitivity, secretion, and elimination in postmenopausal women. Metabolism 2000; 49: 742–747.
Espeland MA, Hogan PE, Fineberg SE, Howard G, Schrott H, Waclawiw MA et al. Effect of postmenopausal hormone therapy on glucose and insulin concentrations. Diabetes Care 1998; 21: 1589–1595.
Acknowledgements
We thank Jon Henderson for expert technical assistance. This work was supported a 4 year studentship from the British Heart Foundation to RSD (FS/07/063/24075). AJD was supported by a Scottish Senior Clinical Fellowship (SCD/09).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Dakin, R., Walker, B., Seckl, J. et al. Estrogens protect male mice from obesity complications and influence glucocorticoid metabolism. Int J Obes 39, 1539–1547 (2015). https://doi.org/10.1038/ijo.2015.102
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.102
This article is cited by
-
Sex-specific effects of a high fat diet on aortic inflammation and dysfunction
Scientific Reports (2023)
-
The metabolic effects of resumption of a high fat diet after weight loss are sex dependent in mice
Scientific Reports (2023)
-
High-fat diet and estrogen impacts the colon and its transcriptome in a sex-dependent manner
Scientific Reports (2020)
-
Androgens sensitise mice to glucocorticoid-induced insulin resistance and fat accumulation
Diabetologia (2019)
-
Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice
Microbiome (2018)