Abstract
Objective:
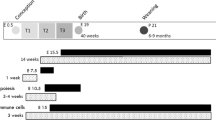
Maternal obesity is associated with increased risk of metabolic dysfunction in the offspring. It is not clear whether it is the metabolic changes or chronic low-grade inflammation in the obese state that causes this metabolic programming. We therefore investigated whether low-grade inflammation was present in obese dams compared with controls dams at gestation day 18 (GD18).
Methods:
Female mice were fed either a standard chow diet or a highly palatable obesogenic diet for 6 weeks before conception. Mice were either kileed before mating (n=12 in each group) or on GD18 (n=8 in each group). Blood and tissues were collected for analysis.
Results:
The obesogenic diet increased body weight and decreased insulin sensitivity before conception, while there was no difference between the groups at GD18. Local inflammation was assayed by macrophage count in adipose tissue (AT) and liver. Macrophage count in the AT was increased significantly by the obesogenic diet, and the hepatic count also showed a tendency to increased macrophage infiltration before gestation. This was further supported by a decreased population of monocytes in the blood of the obese animals, which suggested that monocytes are being recruited from the blood to the liver and AT in the obese animals. Gestation reversed macrophage infiltration, such that obese dams showed a lower AT macrophage count at the end of gestation compared with pre-pregnancy obese mice, and there were no longer a tendency toward increased hepatic macrophage count. Placental macrophage count was also similar in the two groups.
Conclusion:
At GD18, obese dams were found to have similar macrophage infiltration in placenta, AT and liver as lean dams, despite an incipient infiltration before gestation. Thus, the obesity-induced inflammation was reversed during gestation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 2008; 51: 383–392.
Oben JA, Mouralidarane A, Samuelsson AM, Matthews PJ, Morgan ML, McKee C et al. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol 2010; 52: 913–920.
Lumeng CN, Saltiel AR Inflammatory links between obesity and metabolic disease. J Clin Invest 2011; 121: 2111–2117.
Hodyl NA, Krivanek KM, Lawrence E, Clifton VL, Hodgson DM Prenatal exposure to a pro-inflammatory stimulus causes delays in the development of the innate immune response to LPS in the offspring. J Neuroimmunol 2007; 190: 61–71.
Lasala N, Zhou H Effects of maternal exposure to LPS on the inflammatory response in the offspring. J Neuroimmunol 2007; 189: 95–101.
Dahlgren J, Nilsson C, Jennische E, Ho HP, Eriksson E, Niklasson A et al. Prenatal cytokine exposure results in obesity and gender-specific programming. Am J Physiol Endocrinol Metab 2001; 281: E326–E334.
Beloosesky R, Maravi N, Weiner Z, Khatib N, Awad N, Boles J et al. Maternal lipopolysaccharide-induced inflammation during pregnancy programs impaired offspring innate immune responses. Am J Obstet Gynecol 2010; 203: 185 e1–4.
Saito S Cytokine network at the feto-maternal interface. J Reprod Immunol 2000; 47: 87–103.
Saito S Cytokine cross-talk between mother and the embryo/placenta. J Reprod Immunol 2001; 52: 15–33.
Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007; 56: 1761–1772.
Deopurkar R, Ghanim H, Friedman J, Abuaysheh S, Sia CL, Mohanty P et al. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of Toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes Care 2010; 33: 991–997.
Ghanim H, Abuaysheh S, Sia CL, Korzeniewski K, Chaudhuri A, Fernandez-Real JM et al. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care 2009; 32: 2281–2287.
Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC et al. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr 2008; 87: 1219–1223.
Talukdar S, Oh da Y, Bandyopadhyay G, Li D, Xu J, McNelis J et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med 2012; 18: 1407–1412.
Elgazar-Carmon V, Rudich A, Hadad N, Levy R Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res 2008; 49: 1894–1903.
Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med 2009; 15: 940–945.
Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009; 15: 914–920.
Holland WL, Summers SA Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulaton of sphingolipid metabolism. Endocr Rev 2008; 29: 381–402.
Virtue S, Vidal-Puig A Adipose tissue expandability, lipotoxicity and the metabolic syndrome—an allostatic perspective. Biochim Biophys Acta 2010; 1801: 338–349.
Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 2007; 5: 167–179.
Kolak M, Westerbacka J, Velagapudi VR, Wagsater D, Yetukuri L, Makkonen J et al. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes 2007; 56: 1960–1968.
Park TS, Goldberg IJ Sphingolipids, lipotoxic cardiomyopathy, and cardiac failure. Heart Fail Clin 2012; 8: 633-641.
Fernandez-Riejos P, Najib S, Santos-Alvarez J, Martin-Romero C, Perez-Perez A, Gonzalez-Yanes C et al. Role of leptin in the activation of immune cells. Mediators Inflamm 2010; 2010: 568343.
Lumeng CN, Bodzin JL, Saltiel AR Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007; 117: 175–184.
Oben JA, Mouralidarane A, Samuelsson A-M, Matthews PJ, Morgan ML, McKee C et al. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol 2010; 52: 913–920.
Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen E et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance—a novel murine model of developmental programming. Hypertension 2008; 51: 383–392.
Pedersen MH, Lauritzen L, Hellgren LI Fish oil combined with short chain fatty acids synergistically prevent tissue accumulation of free fatty acids during weight loss in obese mice. Br J Nutr 2011; 106: 1449–1456.
Sasaki K, Iwatsuki H Origin and fate of the central macrophages of erythroblastic islands in the fetal and neonatal mouse liver. Microsc Res Techniq 1997; 39: 398–405.
Anderson MJ Permutation tests for univariate or multivariate analysis of variance and regression. Can J Fish Aquat Sci 2001; 58: 626–639.
Holm S A simple sequentially rejective multiple test procedure. Scand J Stat 1979; 6: 65–70.
Fraker DL, Stovroff MC, Merino MJ, Norton JA Tolerance to tumor necrosis factor in rats and the relationship to endotoxin tolerance and toxicity. J Exp Med 1988; 168: 95–105.
Fernandez-Twinn DS, Blackmore HL, Siggens L, Giussani DA, Cross CM, Foo R et al. The programming of cardiac hypertrophy in the offspring by maternal obesity is associated with hyperinsulinemia, AKT, ERK, and mTOR activation. Endocrinology 2012; 153: 5961–5971.
Nixon GF Sphingolipids in inflammation: pathological implications and potential therapeutic targets. Br J Pharmacol 2009; 158: 982–993.
Jiao P, Chen Q, Shah S, Du J, Tao B, Tzameli I et al. Obesity-related upregulation of monocyte chemotactic factors in adipocytes involvement of nuclear factor-kappa B and c-Jun NH2-terminal kinase pathways. Diabetes 2009; 58: 104–115.
Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 2006; 116: 115–124.
Medvedev AE, Vogel SN Overexpression of CD14, TLR4, and MD-2 in HEK 293T cells does not prevent induction of in vitro endotoxin tolerance. J Endotoxin Res 2003; 9: 60–64.
Hauguel-de Mouzon S, Guerre-Millo M The placenta cytokine network and inflammatory signals. Placenta 2006; 27: 794–798.
Lager S, Jansson N, Olsson AL, Wennergren M, Jansson T, Powell TL Effect of IL-6 and TNF-alpha on fatty acid uptake in cultured human primary trophoblast cells. Placenta 2011; 32: 121–127.
Luppi P How immune mechanisms are affected by pregnancy. Vaccine 2003; 21: 3352–3357.
Pazos M, Sperling RS, Moran TM, Kraus TA The influence of pregnancy on systemic immunity. Immunol Res 2012; 54: 254–261.
Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 2008; 29: 274–281.
Robinson DP, Klein SL Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav 2012; 62: 263–271.
Zhu MJ, Ma Y, Long NM, Du M, Ford SP Maternal obesity markedly increases placental fatty acid transporter expression and fetal blood triglycerides at midgestation in the ewe. Am J Physiol-Reg I 2010; 299: R1224–R1231.
McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 2009; 119: 323–335.
Dahlgren J Pregnancy and insulin resistance. Metab Syndr Relat Disord 2006; 4: 149–152.
Shelley P, Martin-Gronert MS, Rowlerson A, Poston L, Heales SJR, Hargreaves IP et al. Altered skeletal muscle insulin signaling and mitochondrial complex II-III linked activity in adult offspring of obese mice. Am J Physiol-Regul Integr Comp Physiol 2009; 297: R675–R681.
Acknowledgements
This study was supported by Otto Moensted foundation 11-70-0597, Aase and Ejnar Danielsen foundation10-000473, Reinholdt W. Jorck and wife’s foundation 11-Ji-0230 and Master of Engineering Frants Allings foundation, the Danish Strategic Research Council through Grant no 09-067124 to ‘Centre for Fetal Programming’ (LIH), as well as the EU FP7 Early Nutrition project Grant no: 289346 to SEO who is also a member of the MRC Metabolic Diseases Unit. Jannie F Agersten and Anne-Marie Nepper are acknowledged for skillful technical assistance in the lipid analysis and Lisbeth Buus Rosholm for cytokine analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
Ingvorsen, C., Thysen, A., Fernandez-Twinn, D. et al. Effects of pregnancy on obesity-induced inflammation in a mouse model of fetal programming. Int J Obes 38, 1282–1289 (2014). https://doi.org/10.1038/ijo.2014.69
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2014.69
Keywords
This article is cited by
-
Differences in lipid metabolism in acquired versus preexisting glucose intolerance during gestation: role of free fatty acids and sphingosine-1-phosphate
Lipids in Health and Disease (2022)
-
Obesity, not a high fat, high sucrose diet alone, induced glucose intolerance and cardiac dysfunction during pregnancy and postpartum
Scientific Reports (2021)
-
Placental Dysfunction Underlies Increased Risk of Fetal Growth Restriction and Stillbirth in Advanced Maternal Age Women
Scientific Reports (2017)
-
Convergence in insulin resistance between very severely obese and lean women at the end of pregnancy
Diabetologia (2015)