Abstract
Background:
A high dietary protein (P) content and low glycemic index (LGI) have been suggested to be beneficial for weight management, but long-term studies are scarce.
Objective:
The DIOGENES randomized clinical trial investigated the effect of P and GI on weight loss maintenance in overweight or obese adults in eight centers across Europe. This study reports the 1-year results in two of the centers that extended the intervention to 1 year.
Method:
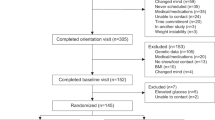
After an 8-week low-calorie diet (LCD), 256 adults (body mass index >27 kg m−2) were randomized to five ad libitum diets for 12 months: high P/LGI (HP/LGI), HP/high GI (HP/HGI), low P/LGI (LP/LGI), LP/HGI and a control diet. During the first 6 months, foods were provided for free through a shop system and during the whole 12-month period, subjects received guidance by a dietician. Primary outcome variable was the change in body weight over the 12-month intervention period.
Results:
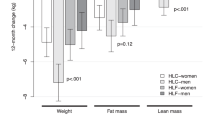
During the LCD period, subjects lost 11.2 (10.8, 12.0) kg (mean (95% confidence interval (CI))). Average weight regain over the 12-month intervention period was 3.9 (95% CI 3.0–4.8) kg. Subjects on the HP diets regained less weight than subjects on the LP diets. The difference in weight regain after 1 year was 2.0 (0.4, 3.6) kg (P=0.017) (completers analysis, N=139) or 2.8 (1.4, 4.1) kg (P<0.001) (intention-to-treat analysis, N=256). No consistent effect of GI on weight regain was found. There were no clinically relevant differences in changes in cardiometabolic risk factors among diet groups.
Conclusion:
A higher protein content of an ad libitum diet improves weight loss maintenance in overweight and obese adults over 12 months.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Skov AR, Toubro S, Ronn B, Holm L, Astrup A . Randomized trial on protein vs carbohydrate in ad libitum fat reduced diet for the treatment of obesity. Int J Obes Relat Metab Disord 1999; 23: 528–536.
Due A, Toubro S, Skov AR, Astrup A . Effect of normal-fat diets, either medium or high in protein, on body weight in overweight subjects: a randomised 1-year trial. Int J Obes Relat Metab Disord 2004; 28: 1283–1290.
Lejeune MP, Kovacs EM, Westerterp-Plantenga MS . Additional protein intake limits weight regain after weight loss in humans. Br J Nutr 2005; 93: 281–289.
Westerterp-Plantenga MS, Lejeune MP, Nijs I, van Ooijen M, Kovacs EM . High protein intake sustains weight maintenance after body weight loss in humans. Int J Obes Relat Metab Disord 2004; 28: 57–64.
Claessens M, van Baak MA, Monsheimer S, Saris WH . The effect of a low-fat, high-protein or high-carbohydrate ad libitum diet on weight loss maintenance and metabolic risk factors. Int J Obes (Lond) 2009; 33: 296–304.
Paddon-Jones D, Westman E, Mattes RD, Wolfe RR, Astrup A, Westerterp-Plantenga M . Protein, weight management, and satiety. Am J Clin Nutr 2008; 87: 1558S–1561S.
Santesso N, Akl EA, Bianchi M, Mente A, Mustafa R, Heels-Ansdell D et al. Effects of higher- versus lower-protein diets on health outcomes: a systematic review and meta-analysis. Eur J Clin Nutr 2012; 66: 780–788.
Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD . Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2012; 96: 1281–1298.
Delbridge EA, Prendergast LA, Pritchard JE, Proietto J . One-year weight maintenance after significant weight loss in healthy overweight and obese subjects: does diet composition matter? Am J Clin Nutr 2009; 90: 1203–1214.
Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009; 360: 859–873.
van Baak MA, Astrup A . Consumption of sugars and body weight. Obes Rev 2009; 10 (Suppl 1): 9–23.
Livesey G, Taylor R, Hulshof T, Howlett J . Glycemic response and health—a systematic review and meta-analysis: relations between dietary glycemic properties and health outcomes. Am J Clin Nutr 2008; 87: 258S–268S.
Schwingshackl L, Hoffmann G . Long-term effects of low glycemic index/load vs high glycemic index/load diets on parameters of obesity and obesity-associated risks: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis 2013; 23: 699–706.
Larsen TM, Dalskov SM, van Baak M, Jebb SA, Papadaki A, Pfeiffer AF et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med 2010; 363: 2102–2113.
Larsen TM, Dalskov S, van Baak M, Jebb S, Kafatos A, Pfeiffer A et al. The Diet, Obesity and Genes (Diogenes) Dietary Study in eight European countries—a comprehensive design for long term intervention. Obes Rev 2010; 11: 76–79.
Saris WH, Astrup A, Prentice AM, Zunft HJ, Formiguera X, Verboeket-van de Venne WP et al. Randomized controlled trial of changes in dietary carbohydrate/fat ratio and simple vs complex carbohydrates on body weight and blood lipids: the CARMEN study. The Carbohydrate Ratio Management in European National diets. Int J Obes Relat Metab Disord 2000; 24: 1310–1318.
Skov AR, Toubro S, Raben A, Astrup A . A method to achieve control of dietary macronutrient composition in ad libitum diets consumed by free-living subjects. Eur J Clin Nutr 1997; 51: 667–672.
Moore CS, Lindroos AK, Kreutzer M, Larsen TM, Astrup A, van Baak MA et al. Strategy to manipulate ad libitum macronutrient intake, and glycaemic index, across eight European countries in the DIOGENES study. Obes Rev 2010; 11: 67–75.
Aston LM, Jackson D, Monsheimer S, Whybrow S, Handjieva-Darlenska T, Kreutzer M et al. Developing a methodology for assigning glycaemic index values to foods consumed across Europe. Obes Rev 2010; 11: 92–100.
Matsuda M, DeFronzo RA . Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22: 1462–1470.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419.
Gogebakan O, Kohl A, Osterhoff MA, van Baak MA, Jebb SA, Papadaki A et al. Effects of weight loss and long-term weight maintenance with diets varying in protein and glycemic index on cardiovascular risk factors: the diet, obesity, and genes (DiOGenes) study: a randomized, controlled trial. Circulation 2011; 124: 2829–2838.
Westerterp KR, Goris AH . Validity of the assessment of dietary intake: problems of misreporting. Curr Opin Clin Nutr Metab Care 2002; 5: 489–493.
Misciagna G, Logroscino G, De Michele G, Cisternino AM, Guerra V, Freudenheim JL . Fructosamine, glycated hemoglobin, and dietary carbohydrates. Clin Chim Acta 2004; 340: 139–147.
Misciagna G, De Michele G, Cisternino AM, Guerra V, Logroscino G, Freudenheim JL . Dietary carbohydrates and glycated proteins in the blood in non diabetic subjects. J Am Coll Nutr 2005; 24: 22–29.
Jenkins DJ, Wolever TM, Collier GR, Ocana A, Rao AV, Buckley G et al. Metabolic effects of a low-glycemic-index diet. Am J Clin Nutr 1987; 46: 968–975.
Acknowledgements
We gratefully acknowledge all food companies for their contributions of foods to the laboratory shops. The DIOGENES trial was funded by the European Commission, contract no. FP6-2005-513946. The funding source had no role in the study design, data collection, data analysis, data interpretation or writing of the report. The majority of the food items in the shops were provided for free by a large number of different food companies. A complete list can be found on www.diogenes-eu.org. Food items to be offered in the shops were selected by the investigators. Companies were in no way involved in the planning, execution or analysis of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Astrup is currently member of advisory boards for McCain Foods, USA, Global Dairy Platform, USA, JennyCraig, USA, and McDonald’s, USA, and has received funding for other studies from about 100 food companies covering all food groups. Dr Saris is corporate scientist, Human Nutrition for DSM, The Netherlands, and received research grants and food donations from several food companies. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Aller, E., Larsen, T., Claus, H. et al. Weight loss maintenance in overweight subjects on ad libitum diets with high or low protein content and glycemic index: the DIOGENES trial 12-month results. Int J Obes 38, 1511–1517 (2014). https://doi.org/10.1038/ijo.2014.52
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2014.52
Keywords
This article is cited by
-
Strategie di mantenimento del calo ponderale nel paziente con obesità
L'Endocrinologo (2023)
-
Effects of foods, beverages and macronutrients on BMI z-score and body composition in children and adolescents: a systematic review and meta-analysis of randomized controlled trials
European Journal of Nutrition (2023)
-
Interaction between the genetic risk score and dietary protein intake on cardiometabolic traits in Southeast Asian
Genes & Nutrition (2020)
-
Protein-Rich Diets for Weight Loss Maintenance
Current Obesity Reports (2020)
-
The role of dietary protein in obesity
Reviews in Endocrine and Metabolic Disorders (2020)