Abstract
Background:
A relationship has been reported between blood concentrations of coagulation factor VII (FVII) and obesity. In addition to its role in coagulation, FVII has been shown to inhibit insulin signals in adipocytes. However, the production of FVII by adipocytes remains unclear.
Objective:
We herein investigated the production and secretion of FVII by adipocytes, especially in relation to obesity-related conditions including adipose inflammation and sympathetic nerve activation.
Methods:
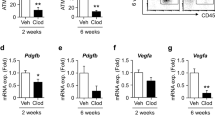
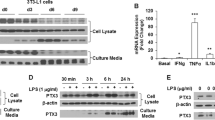
C57Bl/6J mice were fed a low- or high-fat diet and the expression of FVII messenger RNA (mRNA) was then examined in adipose tissue. 3T3-L1 cells were used as an adipocyte model for in vitro experiments in which these cells were treated with tumor necrosis factor-α (TNF-α) or isoproterenol. The expression and secretion of FVII were assessed by quantitative real-time PCR, Western blotting and enzyme-linked immunosorbent assays.
Results:
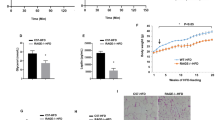
The expression of FVII mRNA in the adipose tissue of mice fed with high-fat diet was significantly higher than that in mice fed with low-fat diet. Expression of the FVII gene and protein was induced during adipogenesis and maintained in mature adipocytes. The expression and secretion of FVII mRNA were increased in the culture medium of 3T3-L1 adipocytes treated with TNF-α, and these effects were blocked when these cells were exposed to inhibitors of mitogen-activated kinases or NF-κB activation. The β-adrenoceptor agonist isoproterenol stimulated the secretion of FVII from mature adipocytes via the cyclic AMP/protein kinase A pathway. Blockade of secreted FVII with the anti-FVII antibody did not affect the phosphorylation of Akt in the isoproterenol-stimulated adipocytes.
Conclusion:
Obese adipose tissue produced FVII. The production and secretion of FVII by adipocytes was enhanced by TNF-α or isoproterenol via different mechanisms. These results indicate that FVII is an adipokine that plays an important role in the pathogenesis of obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Tentolouris N, Liatis S, Katsilambros N . Sympathetic system activity in obesity and metabolic syndrome. Ann NY Acad Sci 2006; 1083: 129–152.
Lambert GW, Straznicky NE, Lambert EA, Dixon JB, Schlaich MP . Sympathetic nervous activation in obesity and the metabolic syndrome—causes, consequences and therapeutic implications. Pharmacol Ther 2010; 126: 159–172.
Canale MP, Manca di Villahermosa S, Martino G, Rovella V, Noce A, De Lorenzo A et al. Obesity-related metabolic syndrome: mechanisms of sympathetic overactivity. Int J Endocrinol 2013; 2013: 865965.
Van Gaal LF, Mertens IL, De Block CE . Mechanisms linking obesity with cardiovascular disease. Nature 2006; 444: 875–880.
Mertens I, Van Gaal LF . Obesity, haemostasis and the fibrinolytic system. Obes Rev 2002; 3: 85–101.
Lorenzet R, Napoleone E, Cutrone A, Donati MB . Thrombosis and obesity: cellular bases. Thromb Res 2012; 129: 285–289.
Monroe DM, Key NS . The tissue factor-factor VIIa complex: procoagulant activity, regulation, and multitasking. J Thromb Haemost 2007; 5: 1097–1105.
Badeanlou L, Furlan-Freguia C, Yang G, Ruf W, Samad F . Tissue factor-protease-activated receptor 2 signaling promotes diet-induced obesity and adipose inflammation. Nat Med 2011; 17: 1490–1497.
Rosito GA, D'Agostino RB, Massaro J, Lipinska I, Mittleman MA, Sutherland P et al. Association between obesity and a prothrombotic state: the Framingham Offspring Study. Thromb Haemost 2004; 91: 683–689.
Godsland IF, Crook D, Proudler AJ, Stevenson JC . Hemostatic risk factors and insulin sensitivity, regional body fat distribution, and the metabolic syndrome. J Clin Endocrinol Metab 2005; 90: 190–197.
Marckmann P, Bladbjerg EM, Jespersen J . Diet and blood coagulation factor VII—a key protein in arterial thrombosis. Eur J Clin Nutr 1998; 52: 75–84.
Connelly JB, Roderick PJ, Cooper JA, Meade TW, Miller GJ . Positive association between self-reported fatty food consumption and factor VII coagulant activity, a risk factor for coronary heart disease, in 4246 middle-aged men. Thromb Haemost 1993; 70: 250–252.
Folsom AR, Qamhieh HT, Wing RR, Jeffery RW, Stinson VL, Kuller LH et al. Impact of weight loss on plasminogen activator inhibitor (PAI-1), factor VII, and other hemostatic factors in moderately overweight adults. Arterioscler Thromb Vasc Biol 1993; 13: 162–169.
Kopp CW, Kopp HP, Steiner S, Kriwanek S, Krzyzanowska K, Bartok A et al. Weight loss reduces tissue factor in morbidly obese patients. Obes Res 2003; 11: 950–956.
Lijnen HR, Van Hul M, Hemmeryckx B . Caloric restriction improves coagulation and inflammation profile in obese mice. Thromb Res 2012; 129: 74–79.
Krysiak R, Gdula-Dymek A, Bachowski R, Okopien B . Pleiotropic effects of atorvastatin and fenofibrate in metabolic syndrome and different types of pre-diabetes. Diabetes Care 2010; 33: 2266–2270.
Dalaker K, Skartlien AH, Prydz H . A new form of coagulation factor VII in plasma. Scand J Haematol 1986; 36: 430–438.
Ceriello A . The post-prandial state and cardiovascular disease: relevance to diabetes mellitus. Diabetes Metab Res Rev 2000; 16: 125–132.
Carr ME . Diabetes mellitus: a hypercoagulable state. J Diabetes Complications 2001; 15: 44–54.
Boden G, Vaidyula VR, Homko C, Cheung P, Rao AK . Circulating tissue factor procoagulant activity and thrombin generation in patients with type 2 diabetes: effects of insulin and glucose. J Clin Endocrinol Metab 2007; 92: 4352–4358.
Rao AK, Chouhan V, Chen X, Sun L, Boden G . Activation of the tissue factor pathway of blood coagulation during prolonged hyperglycemia in young healthy men. Diabetes 1999; 48: 1156–1161.
Kario K, Miyata T, Sakata T, Matsuo T, Kato H . Fluorogenic assay of activated factor VII. Plasma factor VIIa levels in relation to arterial cardiovascular diseases in Japanese. Arterioscler Thromb Vasc Biol 1994; 14: 265–274.
Wilcox JN . Extrahepatic synthesis of factorhuman atherosclerotic vessels VII in human atherosclerotic vessels. Arterioscler Thromb Vasc Biol 2002; 23: 136–141.
Koizume S, Yokota N, Miyagi E, Hirahara F, Nakamura Y, Sakuma Y et al. Hepatocyte nuclear factor-4-independent synthesis of coagulation factor VII in breast cancer cells and its inhibition by targeting selective histone acetyltransferases. Mol Cancer Res 2009; 7: 1928–1936.
Mihara M, Aihara K, Ikeda Y, Yoshida S, Kinouchi M, Kurahashi K et al. Inhibition of thrombin action ameliorates insulin resistance in type 2 diabetic db/db mice. Endocrinology 2010; 151: 513–519.
Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 1996; 84: 491–495.
Takahashi N, Yoshizaki T, Hiranaka N, Suzuki T, Yui T, Akanuma M et al. Suppression of lipin-1 expression increases monocyte chemoattractant protein-1 expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun 2011; 415: 200–205.
Lefterova MI, Lazar MA . New developments in adipogenesis. Trends Endocrinol Metab 2009; 20: 107–114.
Cawthorn WP, Sethi JK . TNF-alpha and adipocyte biology. FEBS Lett 2008; 582: 117–131.
Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S . Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol 2005; 288: H2031–H2041.
Alvarez GE . Sympathetic neural activation in visceral obesity. Circulation 2002; 106: 2533–2536.
Grassi G, Dell’Oro R, Facchini A, Quarti Trevano F, Bolla GB, Mancia G . Effect of central and peripheral body fat distribution on sympathetic and baroreflex function in obese normotensives. J Hypertens 2004; 22: 2363–2369.
Youngstrom TG, Bartness TJ . Catecholaminergic innervation of white adipose tissue in Siberian hamsters. Am J Physiol 1995; 268: R744–R751.
Bartness TJ, Song CK . Thematic review series: adipocyte biology. Sympathetic and sensory innervation of white adipose tissue. J Lipid Res 2007; 48: 1655–1672.
Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK . Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol 2010; 318: 34–43.
Foster MT, Bartness TJ . Sympathetic but not sensory denervation stimulates white adipocyte proliferation. Am J Physiol Regul Integr Comp Physiol 2006; 291: R1630–R1637.
Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S . Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol 2000; 279: C670–C681.
Jimenez M, Barbatelli G, Allevi R, Cinti S, Seydoux J, Giacobino J-P et al. β3-Adrenoceptor knockout in C57BL/6J mice depresses the occurrence of brown adipocytes in white fat. Eur J Biochem 2003; 270: 699–705.
Jackson AA, Cronin KR, Zachariah R, Carew JA . CCAAT/enhancer-binding protein-beta participates in insulin-responsive expression of the factor VII gene. J Biol Chem 2007; 282: 31156–31165.
Cronin KR, Mangan TP, Carew JA . Upregulation of the coagulation factor VII gene during glucose deprivation is mediated by activating transcription factor 4. PLoS One 2012; 7: e40994.
Faber DR, de Groot PG, Visseren FL . Role of adipose tissue in haemostasis, coagulation and fibrinolysis. Obes Rev 2009; 10: 554–563.
Skurk T, Hauner H . Obesity and impaired fibrinolysis: role of adipose production of plasminogen activator inhibitor-1. Int J Obes Relat Metab Disord 2004; 28: 1357–1364.
Halleux CM, Declerck PJ, Tran SL, Detry R, Brichard SM . Hormonal control of plasminogen activator inhibitor-1 gene expression and production in human adipose tissue: stimulation by glucocorticoids and inhibition by catecholamines. J Clin Endocrinol Metab 1999; 84: 4097–4105.
Gottschling-Zeller H, Aprath I, Skurk T, Hauner H . Beta-Adrenoceptor agonists and other cAMP elevating agents suppress PAI-1 production of human adipocytes in primary culture. Horm Metab Res 2000; 32: 509–514.
Acknowledgements
We thank the members of the Department of Biochemistry, School of Dentistry, Health Sciences University of Hokkaido for the use of laboratory equipment. This work was supported in part by JSPS KAKENHI Grant Numbers 25460696, 23590684 (to NT and MI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
OK and TS are employees in Sysmex Corporation.
Rights and permissions
About this article
Cite this article
Takahashi, N., Yoshizaki, T., Hiranaka, N. et al. The production of coagulation factor VII by adipocytes is enhanced by tumor necrosis factor-α or isoproterenol. Int J Obes 39, 747–754 (2015). https://doi.org/10.1038/ijo.2014.208
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2014.208
This article is cited by
-
Generation of fully functional hepatocyte-like organoids from human induced pluripotent stem cells mixed with Endothelial Cells
Scientific Reports (2019)
-
Sarcolipin expression is repressed by endoplasmic reticulum stress in C2C12 myotubes
Journal of Physiology and Biochemistry (2017)
-
Plasma markers of inflammation and hemostatic and endothelial activity in naturally overweight and obese dogs
BMC Veterinary Research (2016)