Abstract
Background/Objectives:
Tissue-specific glucocorticoid metabolism is altered in obesity, and may increase cardiovascular risk. This dysregulation is normalized by short-term calorie restriction and weight loss, an effect that varies with dietary macronutrient composition. However, tissue-specific glucocorticoid metabolism has not been studied during long-term (>6 months) dietary interventions. Therefore our aim was to test whether long-term dietary interventions, either a paleolithic-type diet (PD) or a diet according to Nordic nutrition recommendations (NNR) could normalize tissue-specific glucocorticoid metabolism in overweight and obese women.
Subjects/Methods:
Forty-nine overweight/obese postmenopausal women were randomized to a paleolithic diet or a diet according to NNR for 24 months. At baseline, 6 and 24 months anthropometric measurements, insulin sensitivity, excretion of urinary glucocorticoid metabolites in 24-hour collections, conversion of orally administered cortisone to plasma cortisol and transcript levels of 11β hydroxysteroid dehydrogenase type 1 (11βHSD1) in subcutaneous adipose tissue were studied.
Results:
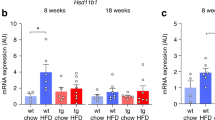
Both diet groups achieved significant and sustained weight loss. Weight loss with the PD was greater than on NNR diet after 6 months (P<0.001) but similar at 24 months. Urinary measurement of 5α-reductase activity was increased after 24 months in both groups compared with baseline (P<0.001). Subcutaneous adipose tissue 11βHSD1 gene expression decreased at 6 and 24 months in both diet groups (P=0.036). Consistent with increased liver 11βHSD1, conversion of oral cortisone to cortisol increased at 6 months (P=0.023) but was unchanged compared with baseline by 24 months.
Conclusions:
Long-term weight loss in postmenopausal women has tissue-specific and time-dependent effects on glucocorticoid metabolism. This may alter local-tissue cortisol exposure contributing to improved metabolic function during weight loss.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Chanson P, Salenave S . Metabolic syndrome in Cushing's syndrome. Neuroendocrinology 2010; 92: 96–101.
Kumari M, Chandola T, Brunner E, Kivimaki M . A nonlinear relationship of generalized and central obesity with diurnal cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab 2010; 95: 4415–4423.
Basu R, Singh RJ, Basu A, Chittilapilly EG, Johnson MC, Toffolo G et al. Obesity and type 2 diabetes do not alter splanchnic cortisol production in humans. J Clin Endocrinol Metab 2005; 90: 3919–3926.
Stimson RH, Andersson J, Andrew R, Redhead DN, Karpe F, Hayes PC et al. Cortisol release from adipose tissue by 11beta-hydroxysteroid dehydrogenase type 1 in humans. Diabetes 2009; 58: 46–53.
Ricketts ML, Verhaeg JM, Bujalska I, Howie AJ, Rainey WE, Stewart PM . Immunohistochemical localization of type 1 11beta-hydroxysteroid dehydrogenase in human tissues. J Clin Endocrinol Metab 1998; 83: 1325–1335.
Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR et al. A transgenic model of visceral obesity and the metabolic syndrome. Science 2001; 294: 2166–2170.
Morton NM, Paterson JM, Masuzaki H, Holmes MC, Staels B, Fievet C et al. Novel adipose tissue-mediated resistance to diet-induced visceral obesity in 11 beta-hydroxysteroid dehydrogenase type 1-deficient mice. Diabetes 2004; 53: 931–938.
Rask E, Olsson T, Soderberg S, Andrew R, Livingstone DE, Johnson O et al. Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab 2001; 86: 1418–1421.
Stimson RH, Andrew R, McAvoy NC, Tripathi D, Hayes PC, Walker BR . Increased whole-body and sustained liver cortisol regeneration by 11beta-hydroxysteroid dehydrogenase type 1 in obese men with type 2 diabetes provides a target for enzyme inhibition. Diabetes 2011; 60: 720–725.
Lottenberg SA, Giannella-Neto D, Derendorf H, Rocha M, Bosco A, Carvalho SV et al. Effect of fat distribution on the pharmacokinetics of cortisol in obesity. Int J Clin Pharmacol Ther 1998; 36: 501–505.
Andrew R, Phillips DI, Walker BR . Obesity and gender influence cortisol secretion and metabolism in man. J Clin Endocrinol Metab 1998; 83: 1806–1809.
Tomlinson JW, Finney J, Gay C, Hughes BA, Hughes SV, Stewart PM . Impaired glucose tolerance and insulin resistance are associated with increased adipose 11beta-hydroxysteroid dehydrogenase type 1 expression and elevated hepatic 5alpha-reductase activity. Diabetes 2008; 57: 2652–2660.
Upreti R, Hughes KA, Livingstone DE, Gray CD, Minns FC, Macfarlane DP et al. 5alpha-Reductase type 1 modulates insulin sensitivity in men. J Clin Endocrinol Metab 2014; 99: 1397–1406.
Dowman JK, Hopkins LJ, Reynolds GM, Armstrong MJ, Nasiri M, Nikolaou N et al. Loss of 5alpha-reductase type 1 accelerates the development of hepatic steatosis but protects against hepatocellular carcinoma in male mice. Endocrinology 2013; 154: 4536–4547.
Johnstone AM, Faber P, Andrew R, Gibney ER, Elia M, Lobley G et al. Influence of short-term dietary weight loss on cortisol secretion and metabolism in obese men. Eur J Endocrinol 2004; 150: 185–194.
Tomlinson JW, Moore JS, Clark PM, Holder G, Shakespeare L, Stewart PM . Weight loss increases 11beta-hydroxysteroid dehydrogenase type 1 expression in human adipose tissue. J Clin Endocrinol Metab 2004; 89: 2711–2716.
Tomlinson JW, Finney J, Hughes BA, Hughes SV, Stewart PM . Reduced glucocorticoid production rate, decreased 5alpha-reductase activity, and adipose tissue insulin sensitization after weight loss. Diabetes 2008; 57: 1536–1543.
Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Heintze U, Janke J et al. Regulation of 11beta-HSD genes in human adipose tissue: influence of central obesity and weight loss. Obes Res 2004; 12: 9–17.
Purnell JQ, Kahn SE, Samuels MH, Brandon D, Loriaux DL, Brunzell JD . Enhanced cortisol production rates, free cortisol, and 11beta-HSD-1 expression correlate with visceral fat and insulin resistance in men: effect of weight loss. Am J Physiol Endocrinol Metab 2009; 296: E351–E357.
Simonyte K, Olsson T, Naslund I, Angelhed JE, Lonn L, Mattsson C et al. Weight loss after gastric bypass surgery in women is followed by a metabolically favorable decrease in 11beta-hydroxysteroid dehydrogenase 1 expression in subcutaneous adipose tissue. J Clin Endocrinol Metab 2010; 95: 3527–3531.
Stimson RH, Mohd-Shukri NA, Bolton JL, Andrew R, Reynolds RM, Walker BR . The post-prandial rise in plasma cortisol in men is mediated by macronutrient-specific stimulation of adrenal and extra-adrenal cortisol production. J Clin Endocrinol Metab 2014; 99: 160–168.
Stimson RH, Johnstone AM, Homer NZ, Wake DJ, Morton NM, Andrew R et al. Dietary macronutrient content alters cortisol metabolism independently of body weight changes in obese men. J Clin Endocrinol Metab 2007; 92: 4480–4484.
Lindeberg S . Food and Western Disease: Health and Nutrition from An Evolutionary Perspective. Wiley Blackwell: Chichester, West Sussex, UK, 2010.
Ryberg M, Sandberg S, Mellberg C, Stegle O, Lindahl B, Larsson C et al. A palaeolithic-type diet causes strong tissue-specific effects on ectopic fat deposition in obese postmenopausal women. J Intern Med 2013; 274: 67–76.
Lindeberg S, Jonsson T, Granfeldt Y, Borgstrand E, Soffman J, Sjostrom K et al. A palaeolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease. Diabetologia 2007; 50: 1795–1807.
Becker W, Lyhne N, Pedersen AN, Aro A, Fogelholm M, Phórsdottir JA et al. Nordic Nutrition Recommendations 2004—integrating nutrition and physical activity. Scand J Nutr 2004; 48: 178–187.
Mellberg C, Sandberg S, Ryberg M, Eriksson M, Brage S, Larsson C et al. Long-term effects of a Palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr 2014; 68: 350–357.
Best R, Walker BR . Additional value of measurement of urinary cortisone and unconjugated cortisol metabolites in assessing the activity of 11 beta-hydroxysteroid dehydrogenase in vivo. Clin Endocrinol (Oxf) 1997; 47: 231–236.
Napolitano A, Voice MW, Edwards CR, Seckl JR, Chapman KE . 11Beta-hydroxysteroid dehydrogenase 1 in adipocytes: expression is differentiation-dependent and hormonally regulated. J Steroid Biochem Mol Biol 1998; 64: 251–260.
Wake DJ, Homer NZ, Andrew R, Walker BR . Acute in vivo regulation of 11beta-hydroxysteroid dehydrogenase type 1 activity by insulin and intralipid infusions in humans. J Clin Endocrinol Metab 2006; 91: 4682–4688.
Sandeep TC, Andrew R, Homer NZ, Andrews RC, Smith K, Walker BR . Increased in vivo regeneration of cortisol in adipose tissue in human obesity and effects of the 11beta-hydroxysteroid dehydrogenase type 1 inhibitor carbenoxolone. Diabetes 2005; 54: 872–879.
Nixon M, Wake DJ, Livingstone DE, Stimson RH, Esteves CL, Seckl JR et al. Salicylate downregulates 11beta-HSD1 expression in adipose tissue in obese mice and in humans, mediating insulin sensitization. Diabetes 2012; 61: 790–796.
Livingstone DE, McInnes KJ, Walker BR, Andrew R . Increased A-ring reduction of glucocorticoids in obese Zucker rats: effects of insulin sensitization. Obes Res 2005; 13: 1523–1526.
Phillipou G . Investigation of urinary steroid profiles as a diagnostic method in Cushing's syndrome. Clin Endocrinol (Oxf) 1982; 16: 433–439.
Basu R, Basu A, Grudzien M, Jung P, Jacobson P, Johnson M et al. Liver is the site of splanchnic cortisol production in obese nondiabetic humans. Diabetes 2009; 58: 39–45.
Basu R, Edgerton DS, Singh RJ, Cherrington A, Rizza RA . Splanchnic cortisol production in dogs occurs primarily in the liver: evidence for substantial hepatic specific 11beta hydroxysteroid dehydrogenase type 1 activity. Diabetes 2006; 55: 3013–3019.
Koska J, de Courten B, Wake DJ, Nair S, Walker BR, Bunt JC et al. 11beta-hydroxysteroid dehydrogenase type 1 in adipose tissue and prospective changes in body weight and insulin resistance. Obesity (Silver Spring) 2006; 14: 1515–1522.
Hughes KA, Manolopoulos KN, Iqbal J, Cruden NL, Stimson RH, Reynolds RM et al. Recycling between cortisol and cortisone in human splanchnic, subcutaneous adipose, and skeletal muscle tissues in vivo. Diabetes 2012; 61: 1357–1364.
Acknowledgements
We thank all of the participants for taking part in this study. We also thank Susanne Sandberg, Inger Arnesjö and Marie Eriksson, Umeå University and the staff of the Wellcome Trust Clinical Research Facility Mass Spectrometry Core, University of Edinburgh. This study was supported by grants from The Swedish Council for Working Life and Social Research (2006-0699 and 2010-0398), the Swedish Research Council (K2011-12237-15-6), the Swedish Heart and Lung Foundation, the County Council of Västerbotten, and Umeå University, Sweden, the British Heart Foundation and the Wellcome Trust.
Clinical trials number: NCT00692536.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
BRW is an inventor on relevant patents owned by the University of Edinburgh. AS, KS, CM, MR, RHS, CL, BL, RA and TO have nothing to disclose.
Rights and permissions
About this article
Cite this article
Stomby, A., Simonyte, K., Mellberg, C. et al. Diet-induced weight loss has chronic tissue-specific effects on glucocorticoid metabolism in overweight postmenopausal women. Int J Obes 39, 814–819 (2015). https://doi.org/10.1038/ijo.2014.188
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2014.188
This article is cited by
-
Effects of very low-calorie ketogenic diet on hypothalamic–pituitary–adrenal axis and renin–angiotensin–aldosterone system
Journal of Endocrinological Investigation (2023)
-
A score appraising Paleolithic diet and the risk of cardiovascular disease in a Mediterranean prospective cohort
European Journal of Nutrition (2022)
-
Influence of Paleolithic diet on anthropometric markers in chronic diseases: systematic review and meta-analysis
Nutrition Journal (2019)
-
Decreased lipogenesis-promoting factors in adipose tissue in postmenopausal women with overweight on a Paleolithic-type diet
European Journal of Nutrition (2018)