Abstract
Objectives:
To examine the specific distribution of liver fat content, visceral and subcutaneous adiposity in normal glucose tolerance (NGT/NGT), isolated impaired fasting glucose (iIFG), isolated impaired glucose tolerance (iIGT) and combined conditions (IFG+IGT), as well as with newly diagnosed type 2 diabetes (nT2D).
Design:
Multicenter, international observational study: cross-sectional analysis.
Subjects:
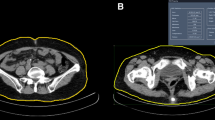
Two thousand five hundred and fifteen patients (50.0% women, 54.5% non-Caucasian) without previously known diabetes were recruited from 29 countries. Abdominal fat distribution was measured by computed tomography (CT). Liver fat was estimated using the CT-liver mean attenuation.
Results:
Compared with NGT/NGT patients, increased visceral adiposity was found in iIFG, iIGT, IFG+IGT and nT2D; estimated liver fat progressively increased across these conditions. A one-s.d. increase in visceral adiposity was associated with an increased risk of having iIFG (men: odds ratio (OR) 1.41 (95% confidence interval (CI) 1.15–1.74), women: OR 1.62 (1.29–2.04)), iIGT (men: OR 1.59 (1.15–2.01), women: OR 1.30 (0.96–1.76)), IFG+IGT (men: OR 1.64 (1.27–2.13), women: OR 1.83 (1.36–2.48)) and nT2D (men: OR 1.80 (1.35–2.42), women: OR 1.73 (1.25–2.41)). A one-s.d. increase in estimated liver fat was associated with iIGT (men: OR 1.46 (1.12–1.90), women: OR 1.81 (1.41–2.35)), IFG+IGT (men: OR 1.42 (1.14–1.77), women: OR 1.74 (1.35–2.26)) and nT2D (men: OR 1.77 (1.40–2.27), women: OR 2.38 (1.81–3.18)). Subcutaneous abdominal adipose tissue showed an inverse relationship with nT2D in women (OR 0.63 (0.45–0.88)).
Conclusions:
Liver fat was associated with iIGT but not with iIFG, whereas visceral adiposity was associated with both. Liver fat and visceral adiposity were associated with nT2D, whereas subcutaneous adiposity showed an inverse relationship with nT2D in women.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hanefeld M, Koehler C, Fuecker K, Henkel E, Schaper F, Temelkova-Kurktschiev T . Insulin secretion and insulin sensitivity pattern is different in isolated impaired glucose tolerance and impaired fasting glucose: the risk factor in Impaired Glucose Tolerance for Atherosclerosis and Diabetes study. Diabetes Care 2003; 26: 868–874.
Tripathy D, Carlsson M, Almgren P, Isomaa B, Taskinen MR, Tuomi T et al. Insulin secretion and insulin sensitivity in relation to glucose tolerance: lessons from the Botnia Study. Diabetes 2000; 49: 975–980.
Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA . Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006; 55: 1430–1435.
Faerch K, Vaag A, Holst JJ, Hansen T, Jorgensen T, Borch-Johnsen K . Natural history of insulin sensitivity and insulin secretion in the progression from normal glucose tolerance to impaired fasting glycemia and impaired glucose tolerance: the Inter99 study. Diabetes Care 2009; 32: 439–444.
Borel AL, Nazare JA, Smith J, Almeras N, Tremblay A, Bergeron J et al. Improvement in insulin sensitivity following a 1-year lifestyle intervention program in viscerally obese men: contribution of abdominal adiposity. Metabolism 2012; 61: 262–272.
Despres JP, Lemieux I . Abdominal obesity and metabolic syndrome. Nature 2006; 444: 881–887.
Hotamisligil GS . Inflammation and metabolic disorders. Nature 2006; 444: 860–867.
Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD . Splanchnic lipolysis in human obesity. J Clin Invest 2004; 113: 1582–1588.
Gastaldelli A, Cusi K, Pettiti M, Hardies J, Miyazaki Y, Berria R et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 2007; 133: 496–506.
Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA 2009; 106: 15430–15435.
van der Zijl NJ, Goossens GH, Moors CC, van Raalte DH, Muskiet MH, Pouwels PJ et al. Ectopic fat storage in the pancreas, liver, and abdominal fat depots: impact on beta-cell function in individuals with impaired glucose metabolism. J Clin Endocrinol Metab 2011; 96: 459–467.
Kantartzis K, Machann J, Schick F, Fritsche A, Haring HU, Stefan N . The impact of liver fat vs visceral fat in determining categories of prediabetes. Diabetologia 2010; 53: 882–889.
Smith JD, Borel AL, Nazare JA, Haffner SM, Balkau B, Ross R et al. Visceral adipose tissue indicates the severity of cardiometabolic risk in patients with and without type 2 diabetes: results from the INSPIRE ME IAA Study. J Clin Endocrinol Metab 2012; 97: 1517–1525.
Good epidemiological practice (GEP) IEA guidelines for proper conduct in epidemiologic research. Available at: http://www.ieaweb.org.
van der Kooy K, Seidell JC . Techniques for the measurement of visceral fat: a practical guide. Int J Obes Relat Metab Disord 1993; 17: 187–196.
Ferland M, Despres JP, Tremblay A, Pinault S, Nadeau A, Moorjani S et al. Assessment of adipose tissue distribution by computed axial tomography in obese women: association with body density and anthropometric measurements. Br J Nutr 1989; 61: 139–148.
Despres JP, Ross R, Boka G, Almeras N, Lemieux I . Effect of rimonabant on the high-triglyceride/ low-HDL-cholesterol dyslipidemia, intraabdominal adiposity, and liver fat: the ADAGIO-Lipids trial. Arterioscler Thromb Vasc Biol 2009; 29: 416–423.
Piekarski J, Goldberg HI, Royal SA, Axel L, Moss AA . Difference between liver and spleen CT numbers in the normal adult: its usefulness in predicting the presence of diffuse liver disease. Radiology 1980; 137: 727–729.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419.
Wareham NJ, Phillips DI, Byrne CD, Hales CN . The 30 minute insulin incremental response in an oral glucose tolerance test as a measure of insulin secretion. Diabet Med 1995; 12: 931.
Abdul-Ghani MA, Tripathy D, DeFronzo RA . Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006; 29: 1130–1139.
Balkau B, Deanfield JE, Despres JP, Bassand JP, Fox KA, Smith SC Jr. et al. International Day for the Evaluation of Abdominal Obesity (IDEA): a study of waist circumference, cardiovascular disease, and diabetes mellitus in 168,000 primary care patients in 63 countries. Circulation 2007; 116: 1942–1951.
Sung KC, Jeong WS, Wild SH, Byrne CD . Combined influence of insulin resistance, overweight/obesity, and fatty liver as risk factors for type 2 diabetes. Diabetes Care 2012; 35: 717–722.
Sung KC, Wild SH, Byrne CD . Resolution of fatty liver and risk of incident diabetes. J Clin Endocrinol Metab 2013; 98: 3637–3643.
Hwang JH, Stein DT, Barzilai N, Cui MH, Tonelli J, Kishore P et al. Increased intrahepatic triglyceride is associated with peripheral insulin resistance: in vivo MR imaging and spectroscopy studies. Am J Physiol Endocrinol Metab 2007; 293: E1663–E1669.
Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 2009; 32: 335–341.
Borel AL, Nazare JA, Smith J, Almeras N, Tremblay A, Bergeron J et al. Visceral and not subcutaneous abdominal adiposity reduction drives the benefits of a 1-year lifestyle modification program. Obesity (Silver Spring) 2012; 20: 1223–1233.
Golan R, Shelef I, Rudich A, Gepner Y, Shemesh E, Chassidim Y et al. Abdominal superficial subcutaneous fat: a putative distinct protective fat subdepot in type 2 diabetes. Diabetes Care 2012; 35: 640–647.
Snijder MB, Visser M, Dekker JM, Goodpaster BH, Harris TB, Kritchevsky SB et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia 2005; 48: 301–308.
Garg A . Acquired and inherited lipodystrophies. N Engl J Med 2004; 350: 1220–1234.
Nazare JA, Smith JD, Borel AL, Haffner SM, Balkau B, Ross R et al. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the International Study of Prediction of Intra-Abdominal Adiposity and Its Relationship With Cardiometabolic Risk/Intra-Abdominal Adiposity. Am J Clin Nutr 2012; 96 (4): 714–726.
van Werven JR, Marsman HA, Nederveen AJ, Smits NJ, ten Kate FJ, van Gulik TM et al. Assessment of hepatic steatosis in patients undergoing liver resection: comparison of US, CT, T1-weighted dual-echo MR imaging, and point-resolved 1H MR spectroscopy. Radiology 2010; 256: 159–168.
Lee SW, Park SH, Kim KW, Choi EK, Shin YM, Kim PN et al. Unenhanced CT for assessment of macrovesicular hepatic steatosis in living liver donors: comparison of visual grading with liver attenuation index. Radiology 2007; 244: 479–485.
Park SH, Kim PN, Kim KW, Lee SW, Yoon SE, Park SW et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology 2006; 239: 105–112.
Ducluzeau PH, Manchec-Poilblanc P, Roullier V, Cesbron E, Lebigot J, Bertrais S et al. Distribution of abdominal adipose tissue as a predictor of hepatic steatosis assessed by MRI. Clin Radiol 2010; 65: 695–700.
Park BJ, Kim YJ, Kim DH, Kim W, Jung YJ, Yoon JH et al. Visceral adipose tissue area is an independent risk factor for hepatic steatosis. J Gastroenterol Hepatol 2008; 23: 900–907.
Acknowledgements
Dr Borel is a postdoctoral fellow supported by a fellowship from Agiràdom (Meylan, France) and from the Rhône-Alpes region (France). Dr Nazare is a postdoctoral fellow supported by a fellowship from the CIHR (Canadian Institute of Health Research). The INSPIRE ME IAA was supported by SANOFI.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
Jean-Pierre Després has received a research grant from Eli Lilly, has received speaker’s fees from Abbott, AstraZeneca, Merck, GlaxoSmithKline and Pfizer Canada Inc. and is on the consultant/advisory board of Novartis, Theratechnologies, Torrent Pharmaceuticals Ltd, Abbott and Sanofi. All the other authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Borel, AL., Nazare, JA., Smith, J. et al. Visceral, subcutaneous abdominal adiposity and liver fat content distribution in normal glucose tolerance, impaired fasting glucose and/or impaired glucose tolerance. Int J Obes 39, 495–501 (2015). https://doi.org/10.1038/ijo.2014.163
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2014.163
This article is cited by
-
Association between MRI-based visceral adipose tissues and metabolic abnormality in a Chinese population: a cross-sectional study
Nutrition & Metabolism (2022)
-
Metabolic and physical function are improved with lifelong 15% calorie restriction in aging male mice
Biogerontology (2022)
-
Visceral Adipose Tissue Inflammation and Radiographic Visceral-to-Subcutaneous Adipose Tissue Ratio in Patients with Cirrhosis
Digestive Diseases and Sciences (2022)
-
Benefit of lifestyle-based T2DM prevention is influenced by prediabetes phenotype
Nature Reviews Endocrinology (2020)
-
Comparison of visceral, general and central obesity indices in the prediction of metabolic syndrome in maintenance hemodialysis patients
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2020)