Abstract
Background/Objectives:
Rates of obesity are greatest in middle age. Obesity is associated with altered activity of brain networks sensing food-related stimuli and internal signals of energy balance, which modulate eating behaviour. The impact of healthy mid-life ageing on these processes has not been characterised. We therefore aimed to investigate changes in brain responses to food cues, and the modulatory effect of meal ingestion on such evoked neural activity, from young adulthood to middle age.
Subjects/Methods:
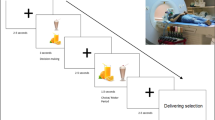
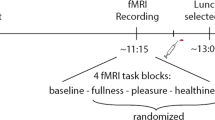
Twenty-four healthy, right-handed subjects, aged 19.5–52.6 years, were studied on separate days after an overnight fast, randomly receiving 50 ml water or 554 kcal mixed meal before functional brain magnetic resonance imaging while viewing visual food cues.
Results:
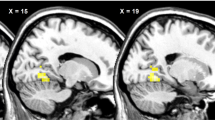
Across the group, meal ingestion reduced food cue-evoked activity of amygdala, putamen, insula and thalamus, and increased activity in precuneus and bilateral parietal cortex. Corrected for body mass index, ageing was associated with decreasing food cue-evoked activation of right dorsolateral prefrontal cortex (DLPFC) and precuneus, and increasing activation of left ventrolateral prefrontal cortex (VLPFC), bilateral temporal lobe and posterior cingulate in the fasted state. Ageing was also positively associated with the difference in food cue-evoked activation between fed and fasted states in the right DLPFC, bilateral amygdala and striatum, and negatively associated with that of the left orbitofrontal cortex and VLPFC, superior frontal gyrus, left middle and temporal gyri, posterior cingulate and precuneus. There was an overall tendency towards decreasing modulatory effects of prior meal ingestion on food cue-evoked regional brain activity with increasing age.
Conclusions:
Healthy ageing to middle age is associated with diminishing sensitivity to meal ingestion of visual food cue-evoked activity in brain regions that represent the salience of food and direct food-associated behaviour. Reduced satiety sensing may have a role in the greater risk of obesity in middle age.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
UN Department of Economic and Social Affairs. World Population Ageing 2009. ESA/P/WP/212 2009; 4–14 Available from http://www.un.org/esa/population/publications/WPA2009/WPA2009_WorkingPaper.pdf.
WHO. Obesity and overweight [Internet]. [cited 2009. October 18]. Available from http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
Flegal KM, Carroll MD, Ogden CL, Curtin LR . Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010; 303: 235–241.
Berthoud H-R, Morrison C . The brain, appetite, and obesity. Annu Rev Psychol. 2008; 59: 55–92.
Trinko R, Sears RM, Guarnieri DJ, DiLeone RJ . Neural mechanisms underlying obesity and drug addiction. Physiol Behav 2007; 91: 499–505.
Le DSNT, Pannacciulli N, Chen K, Del Parigi A, Salbe AD, Reiman EM et al. Less activation of the left dorsolateral prefrontal cortex in response to a meal: a feature of obesity. Am J Clin Nutr 2006; 84: 725–731.
Stoeckel LE, Kim J, Weller RE, Cox JE, Cook EW, Horwitz B . Effective connectivity of a reward network in obese women. Brain Res Bull 2009; 79: 388–395.
Martin LE, Holsen LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR et al. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity (Silver Spring) 2010; 18: 254–260.
Cornier M-A, Salzberg AK, Endly DC, Bessesen DH, Rojas DC, Tregellas JR . The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLoS One 2009; 4: e6310.
Holsen LM, Zarcone JR, Thompson TI, Brooks WM, Anderson MF, Ahluwalia JS et al. Neural mechanisms underlying food motivation in children and adolescents. NeuroImage 2005; 27: 669–676.
Killgore WDS, Yurgelun-Todd DA . Developmental changes in the functional brain responses of adolescents to images of high and low-calorie foods. Dev Psychobiol 2005; 47: 377–397.
Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW . In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci 1999; 2: 859–861.
Dai W, Garcia D, de Bazelaire C, Alsop DC . Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med 2008; 60: 1488–1497.
Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC . Cerebral processing of food-related stimuli: effects of fasting and gender. Behav Brain Res 2006; 169: 111–119.
Flint A, Raben A, Blundell JE, Astrup A . Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 2000; 24: 38–48.
Smith SM . Fast robust automated brain extraction. Hum Brain Mapp 2002; 17: 143–155.
Brett M, Anton J-L, Valabregue R, Poline J-B . Region of interest analysis using an SPM toolbox [abstract]. NeuroImage 2002; 16, Abstract 497.
XBAM version 4.1 Brain Image Analysis Unit, Centre for Neuroimaging Sciences, Institute of Psychiatry, King’s College London. [cited 2011. August 7]. Available from http://www.kcl.ac.uk/ioppn/depts/neuroimaging/research/imaginganalysis/Software/XBAM.aspx.
Brammer MJ, Bullmore ET, Simmons A, Williams SC, Grasby PM, Howard RJ et al. Generic brain activation mapping in functional magnetic resonance imaging: a nonparametric approach. Magn Reson Imaging 1997; 15: 763–770.
Bullmore ET, Brammer MJ, Rabe-Hesketh S, Curtis VA, Morris RG, Williams SC et al. Methods for diagnosis and treatment of stimulus-correlated motion in generic brain activation studies using fMRI. Hum Brain Mapp 1999; 7: 38–48.
Friman O, Borga M, Lundberg P, Knutsson H . Adaptive analysis of fMRI data. NeuroImage. 2003; 19: 837–845.
Bullmore E, Long C, Suckling J, Fadili J, Calvert G, Zelaya F et al. Colored noise and computational inference in neurophysiological (fMRI) time series analysis: resampling methods in time and wavelet domains. Hum Brain Mapp 2001; 12: 61–78.
Talairach J, Tournoux P . Co-Planar Stereotaxic Atlas of the Human Brain: 3-D Proportional System: An Approach to Cerebral Imaging. New York: Thieme, 1998.
Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 2000; 10: 120–131.
Cavanna AE, Trimble MR . The precuneus: a review of its functional anatomy and behavioural correlates. Brain 2006; 129: 564–583.
Djordjevic J, Zatorre RJ, Jones-Gotman M . Effects of perceived and imagined odors on taste detection. Chem Senses 2004; 29: 199–208.
Kringelbach ML, Rolls ET . The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol 2004; 72: 341–372.
Berridge KC, Ho C-Y, Richard JM, DiFeliceantonio AG . The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res 2010; 1350: 43–64.
Fuhrer D, Zysset S, Stumvoll M . Brain activity in hunger and satiety: an exploratory visually stimulated fMRI study. Obesity 2008; 16: 945–950.
Goldstone AP, de Hernandez CGP, Beaver JD, Muhammed K, Croese C, Bell G et al. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci 2009; 30: 1625–1635.
Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, Maravilla K . Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes (Lond) 2009; 33: 653–661.
Pannacciulli N . Le DSNT, Salbe AD, Chen K, Reiman EM, Tataranni PA et al. Postprandial glucagon-like peptide-1 (GLP-1) response is positively associated with changes in neuronal activity of brain areas implicated in satiety and food intake regulation in humans. NeuroImage 2007; 35: 511–517.
Hollmann M, Hellrung L, Pleger B, Schlögl H, Kabisch S, Stumvoll M et al. Neural correlates of the volitional regulation of the desire for food. Int J Obes 2012; 36: 648–655.
Fregni F, Orsati F, Pedrosa W, Fecteau S, Tome FAM, Nitsche MA et al. Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite 2008; 51: 34–41.
Camus M, Halelamien N, Plassmann H, Shimojo S, O’Doherty J, Camerer C et al. Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex decreases valuations during food choices. Eur J Neurosci 2009; 30: 1980–1988.
Smeets PAM, de Graaf C, Stafleu A, van Osch MJP, Nievelstein RAJ, van der Grond J . Effect of satiety on brain activation during chocolate tasting in men and women. Am J Clin Nutr 2006; 83: 1297–1305.
Cornier M-A, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR . Sex-based differences in the behavioral and neuronal responses to food. Physiol Behav 2010; 99: 538–543.
Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM . Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol 2008; 117: 924–935.
Yokum S, Stice E . Cognitive regulation of food craving: effects of three cognitive reappraisal strategies on neural response to palatable foods. Int J Obes (Lond) 2013; 37: 1565–1570.
Smith AB, Halari R, Giampetro V, Brammer M, Rubia K . Developmental effects of reward on sustained attention networks. NeuroImage 2011; 56: 1693–1704.
Ballard IC, Murty VP, Carter RM, MacInnes JJ, Huettel SA, Adcock RA . Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. J Neurosci 2011; 31: 10340–10346.
Hare TA, Camerer CF, Rangel A . Self-control in decision-making involves modulation of the vmPFC valuation system. Science 2009; 324: 646–648.
Van der Laan LN, de Ridder DTD, Viergever MA, Smeets PAM . The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. NeuroImage 2011; 55: 296–303.
Jucaite A, Forssberg H, Karlsson P, Halldin C, Farde L . Age-related reduction in dopamine D1 receptors in the human brain: from late childhood to adulthood, a positron emission tomography study. Neuroscience 2010; 167: 104–110.
Kim I-J, Kim S-J, Kim Y-K . Age- and sex-associated changes in cerebral glucose metabolism in normal healthy subjects: statistical parametric mapping analysis of F-18 fluorodeoxyglucose brain positron emission tomography. Acta Radiol 2009; 50: 1169–1174.
Erixon-Lindroth N, Farde L, Wahlin T-BR, Sovago J, Halldin C, Bäckman L . The role of the striatal dopamine transporter in cognitive aging. Psychiatry Res 2005; 138: 1–12.
Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, MacGregor RR et al. Measuring age-related changes in dopamine D2 receptors with 11C-raclopride and 18F-N-methylspiroperidol. Psychiatry Res 1996; 67: 11–16.
Jacobson A, Green E, Murphy C . Age-related functional changes in gustatory and reward processing regions: an fMRI study. Neuroimage 2010; 53: 602–610.
Green E, Jacobson A, Haase L, Murphy C . Reduced nucleus accumbens and caudate nucleus activation to a pleasant taste is associated with obesity in older adults. Brain Res 2011; 1386: 109–117.
Green E, Jacobson A, Haase L, Murphy C . Can age-related CNS taste differences be detected as early as middle age? Evidence from fMRI. Neuroscience 2013; 232: 194–203.
Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D et al. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs 2000; 32 (Suppl:i–iv): 1–112.
Pijl H, Romijn JA . Obesity, dopamine and the metabolic syndrome: potential of dopaminergic agents in the control of metabolism. Curr Opin Endocrinol Diab 2006; 13: 179–184.
Nummenmaa L, Hirvonen J, Hannukainen JC, Immonen H, Lindroos MM, Salminen P et al. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PLoS One 2012; 7: e31089.
Egecioglu E, Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Jerlhag E et al. Hedonic and incentive signals for body weight control. Rev Endocr Metab Disord 2011; 12: 141–151.
Frank S, Laharnar N, Kullmann S, Veit R, Canova C, Hegner YL et al. Processing of food pictures: influence of hunger, gender and calorie content. Brain Res 2010; 1350: 159–166.
Geliebter A, Pantazatos SP, McOuatt H, Puma L, Gibson CD, Atalayer D . Sex-based fMRI differences in obese humans in response to high vs low energy food cues. Behav Brain Res 2013; 243: 91–96.
Frank TC, Kim GL, Krzemien A, Van Vugt DA . Effect of menstrual cycle phase on corticolimbic brain activation by visual food cues. Brain Res 2010; 1363: 81–92.
Acknowledgements
We acknowledge financial support from King’s College Hospital Charity, Diabetes UK and the UK Department of Health via the National Institute for Health Research comprehensive Biomedical Research Centre award to Guy’s and St Thomas’ Hospitals Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust. We thank research nurses Bula Wilson and Andrew Pernet, the radiographers, and the volunteers who took part.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Cheah, Y., Lee, S., Ashoor, G. et al. Ageing diminishes the modulation of human brain responses to visual food cues by meal ingestion. Int J Obes 38, 1186–1192 (2014). https://doi.org/10.1038/ijo.2013.237
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2013.237
Keywords
This article is cited by
-
The impact of hypoglycaemia awareness status on regional brain responses to acute hypoglycaemia in men with type 1 diabetes
Diabetologia (2018)
-
Altered baseline brain activity differentiates regional mechanisms subserving biological and psychological alterations in obese men
Scientific Reports (2015)