Abstract
Background:
The diet-induced obesity model of zebrafish (DIO-zebrafish) share a common pathophysiological pathway with mammalian obesity.
Objectives:
We aimed to investigate the role of Max dimerization protein 3 (MXD3) in visceral fat accumulation and adipocyte differentiation, by conducting knockdown experiments using zebrafish and mouse preadipocytes.
Methods:
To identify genes related to visceral adiposity, we conducted transcriptome analyses of human and zebrafish obese populations using the Gene Expression Omnibus and DNA microarray. We then intraperitoneally injected morpholino antisense oligonucleotides (MO-mxd3) to knockdown mxd3 gene expression in DIO-zebrafish and measured several parameters, which reflected human obesity and associated metabolic diseases. Finally, lentiviral Mxd3 shRNA knockdown in mouse 3T3-L1 preadipocytes was conducted. Quantitative PCR analyses of several differentiation markers were conducted during these gene knockdown experiments.
Results:
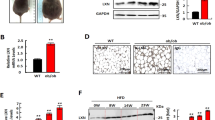
We found that MXD3 expression was increased in the obese population in humans and zebrafish. Intraperitoneal MO-mxd3 administration to DIO-zebrafish suppressed the increase in body weight, visceral fat accumulation and the size of mature adipocytes. Subsequently, dyslipidemia and liver steatosis were also ameliorated by MO-mxd3. In mouse adipocytes, Mxd3 expression was drastically increased in the early differentiation stage. Mxd3 shRNA inhibited preadipocyte proliferation and adipocyte maturation. Quantitative PCR analyses showed that the early differentiation marker, CCAAT/enhancer-binding protein delta (Cebpd) and late differentiation markers (CCAAT/enhancer-binding protein, alpha and peroxisome proliferator-activated receptor gamma) were downregulated by Mxd3 knockdown in 3T3-L1 cells and DIO-zebrafish. Subsequently, mature adipocyte markers (adiponectin and caveolin 1 for zebrafish, and fatty acid binding protein 4 and stearoyl-coenzyme A desaturase 1 for mouse adipocytes) were also decreased.
Conclusion:
Mxd3 regulates preadipocyte proliferation and early adipocyte differentiation via Cebpd downregulation in vitro and in vivo. Integrated analysis of human and zebrafish transcriptomes allows identification of a novel therapeutic target against human obesity and further associated metabolic disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Li C, Ford ES, McGuire LC, Mokdad AH . Increasing trends in waist circumference and abdominal obesity among US adults. Obesity (Silver Spring) 2007; 15: 216–224.
Bessesen DH . Update on obesity. J Clin Endocrinol Metab 2008; 93: 2027–2034.
Ibrahim MM . Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 2010; 11: 11–18.
Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB . Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation 2008; 117: 1658–1667.
Reis JP, Macera CA, Araneta MR, Lindsay SP, Marshall SJ, Wingard DL . Comparison of overall obesity and body fat distribution in predicting risk of mortality. Obesity (Silver Spring) 2009; 17: 1232–1239.
Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ . The biology of white adipocyte proliferation. Obes Rev 2001; 2: 239–254.
Bluher M . Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes 2009; 117: 241–250.
Thorne A, Lonnqvist F, Apelman J, Hellers G, Arner P . A pilot study of long-term effects of a novel obesity treatment: omentectomy in connection with adjustable gastric banding. Int J Obes Relat Metab Disord 2002; 26: 193–199.
Barzilai N, She L, Liu BQ, Vuguin P, Cohen P, Wang J et al. Surgical removal of visceral fat reverses hepatic insulin resistance. Diabetes 1999; 48: 94–98.
Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH et al. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes 2002; 51: 2951–2958.
Lieschke GJ, Currie PD . Animal models of human disease: zebrafish swim into view. Nat Rev Genet 2007; 8: 353–367.
Henderson RJ, Tocher DR . The lipid composition and biochemistry of freshwater fish. Prog Lipid Res 1987; 26: 281–347.
Holtta-Vuori M, Salo VT, Nyberg L, Brackmann C, Enejder A, Panula P et al. Zebrafish: gaining popularity in lipid research. Biochem J 2010; 429: 235–242.
Morais S, Knoll-Gellida A, Andre M, Barthe C, Babin PJ . Conserved expression of alternative splicing variants of peroxisomal acyl-CoA oxidase 1 in vertebrates and developmental and nutritional regulation in fish. Physiol Genomics 2007; 28: 239–252.
Song Y, Cone RD . Creation of a genetic model of obesity in a teleost. FASEB J 2007; 21: 2042–2049.
Stoletov K, Fang L, Choi SH, Hartvigsen K, Hansen LF, Hall C et al. Vascular lipid accumulation, lipoprotein oxidation, and macrophage lipid uptake in hypercholesterolemic zebrafish. Circ Res 2009; 104: 952–960.
Oka T, Nishimura Y, Zang L, Hirano M, Shimada Y, Wang Z et al. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiology 2010; 10: 21.
Tainaka T, Shimada Y, Kuroyanagi J, Zang L, Oka T, Nishimura Y et al. Transcriptome analysis of anti-fatty liver action by Campari tomato using a zebrafish diet-induced obesity model. Nutr Metab (Lond) 2011; 8: 88.
Parichy DM, Mellgren EM, Rawls JF, Lopes SS, Kelsh RN, Johnson SL . Mutational analysis of endothelin receptor b1 (rose) during neural crest and pigment pattern development in the zebrafish Danio rerio. Dev Biol 2000; 227: 294–306.
Westerfield M . A Guide for the Laboratory Use of Zebrafish (Danio rerio) 4th edn. University of Oregon Press: Eugene, OR, 2007.
Hasumura T, Shimada Y, Kuroyanagi J, Nishimura Y, Meguro S, Takema Y et al. Green tea extract suppresses adiposity and affects the expression of lipid metabolism genes in diet-induced obese zebrafish. Nutr Metab (Lond) 2012; 9: 73.
Jones KS, Alimov AP, Rilo HL, Jandacek RJ, Woollett LA, Penberthy WT . A high throughput live transparent animal bioassay to identify non-toxic small molecules or genes that regulate vertebrate fat metabolism for obesity drug development. Nutr Metab (Lond) 2008; 5: 23.
Flynn EJ 3rd, Trent CM, Rawls JF . Ontogeny and nutritional control of adipogenesis in zebrafish (Danio rerio). J Lipid Res 2009; 50: 1641–1652.
Nishio S, Gibert Y, Bernard L, Brunet F, Triqueneaux G, Laudet V . Adiponectin and adiponectin receptor genes are coexpressed during zebrafish embryogenesis and regulated by food deprivation. Dev Dyn 2008; 237: 1682–1690.
Hurlin PJ, Queva C, Koskinen PJ, Steingrimsson E, Ayer DE, Copeland NG et al. Mad3 and Mad4: novel Max-interacting transcriptional repressors that suppress c-myc dependent transformation and are expressed during neural and epidermal differentiation. EMBO J 1995; 14: 5646–5659.
Hardwick KG, Johnston RC, Smith DL, Murray AW . MAD3 encodes a novel component of the spindle checkpoint which interacts with Bub3p, Cdc20p, and Mad2p. J Cell Biol 2000; 148: 871–882.
Queva C, McArthur GA, Iritani BM, Eisenman RN . Targeted deletion of the S-phase-specific Myc antagonist Mad3 sensitizes neuronal and lymphoid cells to radiation-induced apoptosis. Mol Cell Biol 2001; 21: 703–712.
Gore Y, Lantner F, Hart G, Shachar I . Mad3 negatively regulates B cell differentiation in the spleen by inducing Id2 expression. Mol Biol Cell 2010; 21: 1864–1871.
Yun JS, Rust JM, Ishimaru T, Diaz E . A novel role of the Mad family member Mad3 in cerebellar granule neuron precursor proliferation. Mol Cell Biol 2007; 27: 8178–8189.
Barisone GA, Ngo T, Tran M, Cortes D, Shahi MH, Nguyen TV et al. Role of MXD3 in proliferation of DAOY human medulloblastoma cells. PLoS One 2012; 7: e38508.
Pulverer B, Sommer A, McArthur GA, Eisenman RN, Luscher B . Analysis of Myc/Max/Mad network members in adipogenesis: inhibition of the proliferative burst and differentiation by ectopically expressed Mad1. J Cell Physiol 2000; 183: 399–410.
Hermeking H, Wolf DA, Kohlhuber F, Dickmanns A, Billaud M, Fanning E et al. Role of c-myc in simian virus 40 large tumor antigen-induced DNA synthesis in quiescent 3T3-L1 mouse fibroblasts. Proc Natl Acad Sci USA 1994; 91: 10412–10416.
Norton JD, Deed RW, Craggs G, Sablitzky F . Id helix-loop-helix proteins in cell growth and differentiation. Trends Cell Biol 1998; 8: 58–65.
Moldes M, Boizard M, Liepvre XL, Feve B, Dugail I, Pairault J . Functional antagonism between inhibitor of DNA binding (Id) and adipocyte determination and differentiation factor 1/sterol regulatory element-binding protein-1c (ADD1/SREBP-1c) trans-factors for the regulation of fatty acid synthase promoter in adipocytes. Biochem J 1999; 344 (Pt 3): 873–880.
Park KW, Waki H, Villanueva CJ, Monticelli LA, Hong C, Kang S et al. Inhibitor of DNA binding 2 is a small molecule-inducible modulator of peroxisome proliferator-activated receptor-gamma expression and adipocyte differentiation. Mol Endocrinol 2008; 22: 2038–2048.
Hurlin PJ, Huang J . The MAX-interacting transcription factor network. Semin Cancer Biol 2006; 16: 265–274.
Foley KP, McArthur GA, Queva C, Hurlin PJ, Soriano P, Eisenman RN . Targeted disruption of the MYC antagonist MAD1 inhibits cell cycle exit during granulocyte differentiation. EMBO J 1998; 17: 774–785.
Schreiber-Agus N, Meng Y, Hoang T, Hou H Jr., Chen K, Greenberg R et al. Role of Mxi1 in ageing organ systems and the regulation of normal and neoplastic growth. Nature 1998; 393: 483–487.
Heath VJ, Gillespie DA, Crouch DH . Inhibition of the terminal stages of adipocyte differentiation by cMyc. Exp Cell Res 2000; 254: 91–98.
Cao Z, Umek RM, McKnight SL . Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev 1991; 5: 1538–1552.
Wu Z, Bucher NL, Farmer SR . Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol Cell Biol 1996; 16: 4128–4136.
Niehof M, Manns MP, Trautwein C . CREB controls LAP/C/EBP beta transcription. Mol Cell Biol 1997; 17: 3600–3613.
Reusch JE, Colton LA, Klemm DJ . CREB activation induces adipogenesis in 3T3-L1 cells. Mol Cell Biol 2000; 20: 1008–1020.
Si J, Yu X, Zhang Y, DeWille JW . Myc interacts with Max and Miz1 to repress C/EBPdelta promoter activity and gene expression. Mol Cancer 2010; 9: 92.
Eberle D, Hegarty B, Bossard P, Ferre P, Foufelle F . SREBP transcription factors: master regulators of lipid homeostasis. Biochimie 2004; 86: 839–848.
Edgar R, Domrachev M, Lash AE . Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 2002; 30: 207–210.
Acknowledgements
We thank S Ichikawa for her experimental assistance; A Suzuki for zebrafish breeding and maintenance; and R Ikeyama and Y Tamura for secretarial assistance. This work was supported in part by a KAKENHI (23659136) Grant-in-Aid for Scientific Research, Science and Technology Agency, and the New Energy and Industrial Technology Development Organization in Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Shimada, Y., Kuroyanagi, J., Zhang, B. et al. Downregulation of Max dimerization protein 3 is involved in decreased visceral adipose tissue by inhibiting adipocyte differentiation in zebrafish and mice. Int J Obes 38, 1053–1060 (2014). https://doi.org/10.1038/ijo.2013.217
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2013.217
Keywords
This article is cited by
-
Development of a Novel Zebrafish Model for Type 2 Diabetes Mellitus
Scientific Reports (2017)
-
E2F8 promotes hepatic steatosis through FABP3 expression in diet-induced obesity in zebrafish
Nutrition & Metabolism (2015)