Abstract
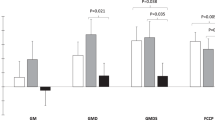
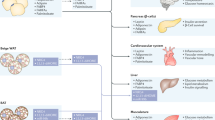
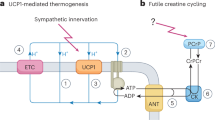
Subcutaneous adipose tissue represents about 85% of all body fat. Its major metabolic role is the regulated storage and mobilization of lipid energy. It stores lipid in the form of triacylglycerol (TG), which is mobilized, as required for use by other tissues, in the form of non-esterified fatty acids (NEFA). Neither TG nor NEFA are soluble to any extent in water, and their transport to and out of the tissue requires specialized transport mechanisms and adequate blood flow. Subcutaneous adipose tissue blood flow (ATBF) is therefore tightly linked to the tissue’s metabolic functioning. ATBF is relatively high (in the fasting state, similar to that of resting skeletal muscle, when expressed per 100 g tissue) and changes markedly in different physiological states. Those most studied are after ingestion of a meal, when there is normally a marked rise in ATBF, and exercise, when ATBF also increases. Pharmacological studies have helped to define the physiological regulation of ATBF. Adrenergic influences predominate in most situations, but nevertheless the regulation of ATBF is complex and depends on the interplay of many different systems. ATBF is downregulated in obesity (when expressed per 100 g tissue), and its responsiveness to meal intake is reduced. However, there is little evidence that this leads to adipose tissue hypoxia in human obesity, and we suggest that, like the downregulation of catecholamine-stimulated lipolysis seen in obesity, the reduction in ATBF represents an adaptation to the increased fat mass. Most information on ATBF has been obtained from studying the subcutaneous abdominal fat depot, but more limited information on lower-body fat depots suggests some similarities, but also some differences: in particular, marked alpha-adrenergic tone, which can reduce the femoral ATBF response to adrenergic stimuli.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Thomas EL, Saeed N, Hajnal JV, Brynes A, Goldstone AP, Frost G et al. Magnetic resonance imaging of total body fat. J Appl Physiol 1998; 85: 1778–1785.
Frayn KN, Macdonald IA . Adipose tissue circulation. In: Bennett T, Gardiner SM, (eds). Nervous Control of Blood Vessels 1996. Harwood Academic: Amsterdam pp 505–539.
Sotornik R, Brassard P, Martin E, Yale P, Carpentier AC, Ardilouze JL . Update on adipose tissue blood flow regulation. Am J Physiol Endocrinol Metab 2012; 302: E1157–E1170.
Lee MJ, Wu Y, Fried SK . Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med 2013; 34: 1–11.
Alemany M . Regulation of adipose tissue energy availability through blood flow control in the metabolic syndrome. Free Radic Biol Med 2012; 52: 2108–2119.
Goossens GH, Blaak EE . Adipose tissue oxygen tension: implications for chronic metabolic and inflammatory diseases. Curr Opin Clin Nutr Metab Care 2012; 15: 539–546.
Thompson D, Karpe F, Lafontan M, Frayn K . Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol Rev 2012; 92: 157–191.
Berthoud H-R, Fox EA, Neuhuber WL . Vagaries of adipose tissue innervation. Am J Physiol Regul Integr Comp Physiol 2006; 291: R1240–R1242.
Kreier F, Buijs RM . Evidence for parasympathetic innervation of white adipose tissue, clearing up some vagaries. Am J Physiol Regul Integr Comp Physiol 2007; 293: R548–R549.
Bickerton AS, Roberts R, Fielding BA, Hodson L, Blaak EE, Wagenmakers AJ et al. Preferential uptake of dietary fatty acids in adipose tissue and muscle in the postprandial period. Diabetes 2007; 56: 168–176.
Hodson L, Skeaff CM, Fielding BA . Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Progr Lipid Res 2008; 47: 348–380.
Strawford A, Antelo F, Christiansen M, Hellerstein MK . Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. Am J Physiol Endocrinol Metab 2004; 286: E577–E588.
Pinnick KE, Neville MJ, Fielding BA, Frayn KN, Karpe F, Hodson L . Gluteofemoral adipose tissue plays a major role in production of the lipokine palmitoleate in humans. Diabetes 2012; 61: 1399–4103.
Collins JM, Neville MJ, Pinnick KE, Hodson L, Ruyter B, van Dijk TH et al. De novo lipogenesis in the differentiating human adipocyte can provide all fatty acids necessary for maturation. J Lipid Res 2011; 52: 1683–1692.
Camps L, Reina M, Llobera M, Vilar S, Olivecrona T . Lipoprotein lipase: cellular origin and functional distribution. Amer J Physiol 1990; 258: C673–C681.
Wang H, Eckel RH . Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab 2009; 297: E271–E288.
Fielding BA, Frayn KN . Lipoprotein lipase and the disposition of dietary fatty acids. Brit J Nutr 1998; 80: 495–502.
Ruge T, Hodson L, Cheeseman J, Dennis AL, Fielding BA, Humphreys SM et al. Fasted to fed trafficking of fatty acids in human adipose tissue reveals a novel regulatory step for enhanced fat storage. J Clin Endocrinol Metab 2009; 94: 1781–1788.
Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A et al. FAT SIGNALS—lipases and lipolysis in lipid metabolism and signaling. Cell Metab 2012; 15: 279–291.
Lafontan M, Langin D . Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res 2009; 48: 275–297.
Samra JS, Simpson EJ, Clark ML, Forster CD, Humphreys SM, Macdonald IA et al. Effects of epinephrine infusion on adipose tissue: interactions between blood flow and lipid metabolism. Am J Physiol 1996; 271: E834–E839.
Goossens GH, Karpe F . Human adipose tissue blood flow and micromanipulation of human subcutaneous blood flow. Methods Mol Biol 2008; 456: 97–107.
Bülow J . Measurement of adipose tissue blood flow. Methods Mol Biol 2001; 155: 281–293.
Larsen OA, Lassen NA, Quaade F . Blood flow through human adipose tissue determined with radioactive xenon. Acta Physiol Scand 1966; 66: 337–345.
Samra JS, Frayn KN, Giddings JA, Clark ML, Macdonald IA . Modification and validation of a commercially available portable detector for measurement of adipose tissue blood flow. Clin Physiol 1995; 15: 241–248.
Bülow J, Jelnes R, Astrup A, Madsen J, Vilmann P . Tissue/blood partition coefficients for xenon in various adipose tissue depots in man. Scand J Clin Lab Invest 1987; 47: 1–3.
Jelnes R, Rasmussen LB, Eickhoff JH . Direct determination of the tissue-to-blood partition coefficient for Xenon in human subcutaneous adipose tissue. Scand J Clin Lab Invest 1984; 44: 643–647.
Simonsen L, Bülow J, Astrup A, Madsen J, Christensen NJ . Diet-induced changes in subcutaneous adipose tissue blood flow in man: effect of ß-adrenoceptor inhibition. Acta Physiol Scand 1990; 139: 341–346.
Karpe F, Fielding BA, Ardilouze J-L, Ilic V, Macdonald IA, Frayn KN . Effects of insulin on adipose tissue blood flow in man. J Physiol 2002; 540: 1087–1093.
Ardilouze J, Fielding B, Currie J, Frayn K, Karpe F . Nitric oxide and beta-adrenergic stimulation are major regulators of pre- and postprandial subcutaneous adipose tissue blood flow in humans. Circulation 2004; 109: 47–52.
Goossens GH, McQuaid SE, Dennis AL, van Baak MA, Blaak EE, Frayn KN et al. Angiotensin II: a major regulator of subcutaneous adipose tissue blood flow in humans. J Physiol 2006; 571: 451–460.
Ardilouze JL, Sotornik R, Dennis LA, Fielding BA, Frayn KN, Karpe F . Failure to increase postprandial blood flow in subcutaneous adipose tissue is associated with tissue resistance to adrenergic stimulation. Diabetes Metab 2012; 38: 27–33.
Galitzky J, Lafontan M, Nordenström J, Arner P . Role of vascular alpha-2 adrenoceptors in regulating lipid mobilization from human adipose tissue. J Clin Invest 1993; 91: 1997–2003.
Barbe P, Millet L, Galitzky J, Lafontan M, Berlan M . In situ assessment of the role of the beta 1-, beta 2- and beta 3-adrenoceptors in the control of lipolysis and nutritive blood flow in human subcutaneous adipose tissue. Br J Pharmacol 1996; 117: 907–913.
Barbe P, Stich V, Galitzky J, Kunesova M, Hainer V, Lafontan M et al. In vivo increase in beta-adrenergic lipolytic response in subcutaneous adipose tissue of obese subjects submitted to a hypocaloric diet. J Clin Endocrinol Metab 1997; 82: 63–69.
Galitzky J, Sengenès C, Thalamas C, Marques MA, Senard JM, Lafontan M et al. The lipid-mobilizing effect of atrial natriuretic peptide is unrelated to sympathetic nervous system activation or obesity in young men. J Lipid Res 2001; 42: 536–544.
Karpe F, Fielding BA, Ilic V, Humphreys SM, Frayn KN . Monitoring adipose tissue blood flow in man: a comparison between the 133xenon washout method and microdialysis. Int J Obes 2002; 26: 1–5.
Viljanen AP, Lautamäki R, Jarvisalo M, Parkkola R, Huupponen R, Lehtimäki T et al. Effects of weight loss on visceral and abdominal subcutaneous adipose tissue blood-flow and insulin-mediated glucose uptake in healthy obese subjects. Ann Med 2009; 41: 152–160.
Iozzo P, Viljanen A, Guzzardi MA, Laine H, Honka MJ, Ferrannini E et al. The interaction of blood flow, insulin, and bradykinin in regulating glucose uptake in lower-body adipose tissue in lean and obese subjects. J Clin Endocrinol Metab 2012; 97: E1192–E1196.
Wellhöner P, Rolle D, Lönnroth P, Strindberg L, Elam M, Dodt C . Laser-Doppler flowmetry reveals rapid perfusion changes in adipose tissue of lean and obese females. Am J Physiol Endocrinol Metab 2006; 291: E1025–E1030.
Tobin L, Simonsen L, Bülow J . Real-time contrast-enhanced ultrasound determination of microvascular blood volume in abdominal subcutaneous adipose tissue in man. Evidence for adipose tissue capillary recruitment. Clin Physiol Funct Imaging 2010; 30: 447–452.
Tobin L, Simonsen L, Bülow J . The dynamics of the microcirculation in the subcutaneous adipose tissue is impaired in the postprandial state in type 2 diabetes. Clin Physiol Funct Imaging 2011; 31: 458–463.
Flechtner-Mors M, Jenkinson CP, Alt A, Adler G, Ditschuneit HH . In vivo α1-adrenergic lipolytic activity in subcutaneous adipose tissue of obese subjects. J Pharmacol Exp Ther 2002; 301: 229–233.
Manolopoulos KN, Karpe F, Frayn KN . Marked resistance of femoral adipose tissue blood flow and lipolysis to adrenaline in vivo. Diabetologia 2012; 55: 3029–3037.
Birkenfeld AL, Boschmann M, Moro C, Adams F, Heusser K, Franke G et al. Lipid mobilization with physiological atrial natriuretic peptide concentrations in humans. J Clin Endocrinol Metab 2005; 90: 3622–3628.
Arner P, Hellmér J, Hagström-Toft E, Bolinder J . Effect of phosphodiesterase inhibition with amrinone or theophylline on lipolysis and blood flow in human adipose tissue in vivo as measured with microdialysis. J Lipid Res 1993; 34: 1737–1743.
Flechtner-Mors M, Jenkinson CP, Alt A, Biesalski HK, Adler G, Ditschuneit HH . Studies of phosphodiesterase effects on adipose tissue metabolism in obese subjects by the microdialysis technique. J Physiol Pharmacol 2005; 56: 355–368.
Coppack SW, Evans RD, Fisher RM, Frayn KN, Gibbons GF, Humphreys SM et al. Adipose tissue metabolism in obesity: lipase action in vivo before and after a mixed meal. Metabolism 1992; 41: 264–272.
Summers LKM, Samra JS, Humphreys SM, Morris RJ, Frayn KN . Subcutaneous abdominal adipose tissue blood flow: variation within and between subjects and relationship to obesity. Clin Sci 1996; 91: 679–683.
Karpe F, Fielding BA, Ilic V, Macdonald IA, Summers LKM, Frayn KN . Impaired postprandial adipose tissue blood flow response is related to aspects of insulin sensitivity. Diabetes 2002; 51: 2467–2473.
Fried SK, Russell CD, Grauso NL, Brolin RE . Lipoprotein lipase regulation by insulin and glucocorticoid in subcutaneous and omental adipose tissues of obese women and men. J Clin Invest 1993; 92: 2191–2198.
Hjemdahl P, Linde B . Influence of circulating NE and Epi on adipose tissue vascular resistance and lipolysis in humans. Am J Physiol 1983; 245: H447–H452.
Patel JN, Eisenhofer G, Coppack SW, Miles JM . Norepinephrine spillover in forearm and subcutaneous adipose tissue before and after eating. J Clin Endocrinol Metab 1999; 84: 2815–2819.
Perez-Matute P, Neville MJ, Tan GD, Frayn KN, Karpe F . Transcriptional control of human adipose tissue blood flow. Obesity 2009; 17: 681–688.
Bertin E, Arner P, Bolinder J, Hagström-Toft E . Action of glucagon and glucagon-like peptide-1-(7-36) amide on lipolysis in human subcutaneous adipose tissue and skeletal muscle in vivo. J Clin Endocrinol Metab 2001; 86: 1229–1234.
Asmar M, Simonsen L, Madsbad S, Stallknecht B, Holst JJ, Bülow J . Glucose-dependent insulinotropic polypeptide may enhance fatty acid re-esterification in subcutaneous abdominal adipose tissue in lean humans. Diabetes 2010; 59: 2160–2163.
van Hall G, Bülow J, Sacchetti M, Al Mulla N, Lyngsø D, Simonsen L . Regional fat metabolism in human splanchnic and adipose tissues; the effect of exercise. J Physiol 2002; 543: 1033–1046.
Hodgetts V, Coppack SW, Frayn KN . Hockaday TDR Factors controlling fat mobilization from human subcutaneous adipose tissue during exercise. J Appl Physiol 1991; 71: 445–451.
Lange KH, Lorentsen J, Isaksson F, Simonsen L, Juul A, Christensen NJ et al. Subcutaneous abdominal adipose tissue lipolysis during exercise determined by arteriovenous measurements in older women. J Am Geriatr Soc 2002; 50: 275–281.
Bülow J, Madsen J . Adipose tissue blood flow during prolonged, heavy exercise. Pflügers Arch 1976; 363: 231–234.
Heinonen I, Bucci M, Kemppainen J, Knuuti J, Nuutila P, Boushel R et al. Regulation of subcutaneous adipose tissue blood flow during exercise in humans. J Appl Physiol 2012; 112: 1059–1063.
Bülow J . Human adipose tissue blood flow during prolonged exercise, III. Effect of β-adrenergic blockade, nicotinic acid and glucose infusion. Scand J Clin Lab Invest 1981; 41: 415–424.
Moro C, Crampes F, Sengenes C, De Glisezinski I, Galitzky J, Thalamas C et al. Atrial natriuretic peptide contributes to physiological control of lipid mobilization in humans. FASEB J 2004; 18: 908–910.
Moro C, Pillard F, De Glisezinski I, Harant I, Riviere D, Stich V et al. Training enhances ANP lipid-mobilizing action in adipose tissue of overweight men. Med Sci Sports Exerc 2005; 37: 1126–1132.
Frayn KN, Humphreys SM . Metabolic characteristics of human subcutaneous abdominal adipose tissue after overnight fast. Am J Physiol Endocrinol Metab 2012; 302: E468–E475.
Langin D, Dicker A, Tavernier G, Hoffstedt J, Mairal A, Rydén M et al. Adipocyte lipases and defect of lipolysis in human obesity. Diabetes 2005; 54: 3190–3197.
McQuaid SE, Hodson L, Neville MJ, Dennis AL, Cheeseman J, Humphreys SM et al. Down-regulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes 2011; 60: 47–55.
Frayn KN . Obesity and metabolic disease: is adipose tissue the culprit? Proc Nutr Soc 2005; 64: 7–13.
Stich V, De Glisezinski I, Crampes F, Hejnova J, Cottet-Emard JM, Galitzky J et al. Activation of α2-adrenergic receptors impairs exercise-induced lipolysis in SCAT of obese subjects. Am J Physiol Regul Integr Comp Physiol 2000; 279: R499–R504.
Stich V, Marion Latard F, Hejnova J, Viguerie N, Lefort C, Suljkovicova H et al. Hypocaloric diet reduces exercise-induced alpha 2-adrenergic antilipolytic effect and alpha 2-adrenergic receptor mRNA levels in adipose tissue of obese women. J Clin Endocrinol Metab 2002; 87: 1274–1281.
Jansson PA, Larsson A, Lonnroth PN . Relationship between blood pressure, metabolic variables and blood flow in obese subjects with or without non-insulin-dependent diabetes mellitus. Eur J Clin Invest 1998; 28: 813–818.
Mitrou P, Boutati E, Lambadiari V, Maratou E, Komesidou V, Papakonstantinou A et al. Rates of lipid fluxes in adipose tissue in vivo after a mixed meal in morbid obesity. Int J Obes 2010; 34: 770–774.
Dimitriadis G, Lambadiari V, Mitrou P, Maratou E, Boutati E, Panagiotakos DB et al. Impaired postprandial blood flow in adipose tissue may be an early marker of insulin resistance in type 2 diabetes. Diabetes Care 2007; 30: 3128–3130.
Trayhurn P . Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev 2013; 93: 1–21.
Pasarica M, Rood J, Ravussin E, Schwarz JM, Smith SR, Redman LM . Reduced oxygenation in human obese adipose tissue is associated with impaired insulin suppression of lipolysis. J Clin Endocrinol Metab 2010; 95: 4052–4055.
Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E et al. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation 2011; 124: 67–76.
Hodson L, Humphreys SM, Karpe F, Frayn KN . Metabolic signatures of human adipose tissue hypoxia in obesity. Diabetes 2013; 62: 1417–1425.
Manolopoulos KN, Karpe F, Frayn KN . Gluteofemoral body fat as a determinant of metabolic health. Int J Obes 2010; 34: 949–959.
Wahrenberg H, Lönnqvist F, Arner P . Mechanisms underlying regional differences in lipolysis in human adipose tissue. J Clin Invest 1989; 84: 458–467.
Arner P, Kriegholm E, Engfeldt P, Bolinder J . Adrenergic regulation of lipolysis in situ at rest and during exercise. J Clin Invest 1990; 85: 893–898.
Jensen MD . Lipolysis: contribution from regional fat. Annu Rev Nutr 1997; 17: 127–139.
Tan G, Goossens GH, Humphreys SM, Vidal H, Karpe F . Upper and lower body adipose tissue function: a direct comparison of fat mobilization in humans. Obes Res 2004; 12: 114–118.
McQuaid SE, Humphreys SM, Hodson L, Fielding BA, Karpe F, Frayn KN . Femoral adipose tissue may accumulate the fat that has been recycled as VLDL and nonesterified fatty acids. Diabetes 2010; 59: 2465–2473.
Millet L, Barbe P, Lafontan M, Berlan M, Galitzky J . Catecholamine effects on lipolysis and blood flow in human abdominal and femoral adipose tissue. J Appl Physiol 1998; 85: 181–188.
Jansson P-A, Larsson A, Smith U, Lönnroth P . Glycerol production in subcutaneous adipose tissue in lean and obese humans. J Clin Invest 1992; 89: 1610–1617.
Acknowledgements
We thank many colleagues who have worked with us on ATBF regulation, including Professor Jean-Luc Ardilouze from the University of Sherbrooke and Dr Gijs Goossens from Maastricht University. We have particularly used data generated by Dr Siobhán McQuaid and Dr Konstantinos Manolopoulos in writing this review. Funding for our work in this area has come from many sources including British Heart Foundation, Biotechnology and Biological Sciences Research Council and Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Frayn, K., Karpe, F. Regulation of human subcutaneous adipose tissue blood flow. Int J Obes 38, 1019–1026 (2014). https://doi.org/10.1038/ijo.2013.200
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2013.200
Keywords
This article is cited by
-
Regulatory T cells in skin regeneration and wound healing
Military Medical Research (2023)
-
The evolving functions of the vasculature in regulating adipose tissue biology in health and obesity
Nature Reviews Endocrinology (2023)
-
Obesity and Peripheral Artery Disease: Current Evidence and Controversies
Current Obesity Reports (2023)
-
SubQ-Sim: A Subcutaneous Physiologically Based Biopharmaceutics Model. Part 1: The Injection and System Parameters
Pharmaceutical Research (2023)
-
Long-term hematopoietic stem cells as a parasite niche during treatment failure in visceral leishmaniasis
Communications Biology (2022)