Abstract
Background:
The transcription factor SIM1 (Single-minded 1) is involved in the control of food intake and in the pathogenesis of obesity. In mice, Sim1 is involved in the development of the paraventricular nucleus, and Sim1 deficiency leads to severe obesity and hyperphagia. In humans, chromosomal abnormalities in the SIM1 gene region have been reported in obese individuals. Furthermore, recent data also suggest that loss-of-function point mutations in SIM1 are responsible for SIM1 haplo-insufficiency that is involved in causing human obesity. In this study, we therefore wanted to expand the evidence regarding the involvement of SIM1 mutations in the pathogenesis of severe early-onset obesity.
Methods:
We screened 561 severely overweight and obese children and adolescents and 453 lean adults for mutations in the coding region of the SIM1 gene. Mutation screening in all patients and lean individuals was performed by high-resolution melting curve analysis combined with direct sequencing. To evaluate the effect of the mutations on SIM1 transcriptional activity, luciferase reporter assays were performed.
Results:
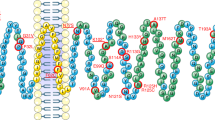
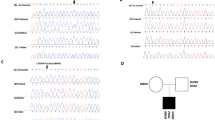
Mutation analysis identified four novel nonsynonymous coding variants in SIM1 in four unrelated obese individuals: p.L242V, p.T481K, p.A517V and p.D590E. Five synonymous variants, p.P57P, p.F93F, p.I183I, p.V208V and p.T653T, were also identified. Screening of the lean control population revealed the occurrence of four other rare SIM1 variants: p.G408R, p.R471P, p.S492P and p.S622F. For variants p.T481K and p.A517V, which were found in obese individuals, a decrease in SIM1 transcriptional activity was observed, whereas the transcriptional activity of all variants found in lean individuals resembled wild type.
Conclusions:
In this study, we have demonstrated the presence of rare SIM1 variants in both an obese pediatric population and a population of lean adult controls. Further, we have shown that functional in vitro analysis of SIM1 variants may help in distinguishing benign variants of no pathogenic significance from variants which contribute to the obesity phenotype.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Accession codes
References
Michaud JL, Rosenquist T, May NR, Fan CM . Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev 1998; 12: 3264–3275.
Palkovits M, Mezey E, Eskay RL . Pro-opiomelanocortin-derived peptides (ACTH/beta-endorphin/alpha-MSH) in brainstem baroreceptor areas of the rat. Brain Res 1987; 436: 323–338.
Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD . Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol 1994; 8: 1298–1308.
Michaud JL, Boucher F, Melnyk A, Gauthier F, Goshu E, Levy E et al. Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Hum Mol Genet 2001; 10: 1465–1473.
Sims JS, Lorden JF . Effect of paraventricular nucleus lesions on body weight, food intake and insulin levels. Behav Brain Res 1986; 22: 265–281.
Tolson KP, Gemelli T, Gautron L, Elmquist JK, Zinn AR, Kublaoui BM . Postnatal Sim1 deficiency causes hyperphagic obesity and reduced Mc4r and oxytocin expression. J Neurosci 2010; 30: 3803–3812.
Kublaoui BM, Holder JL Jr, Tolson KP, Gemelli T, Zinn AR . SIM1 overexpression partially rescues agouti yellow and diet-induced obesity by normalizing food intake. Endocrinology 2006; 147: 4542–4549.
Holder JL Jr, Butte NF, Zinn AR . Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum Mol Genet 2000; 9: 101–108.
Faivre L, Cormier-Daire V, Lapierre JM, Colleaux L, Jacquemont S, Genevieve D et al. Deletion of the SIM1 gene (6q16.2) in a patient with a Prader-Willi-like phenotype. J Med Genet 2002; 39: 594–596.
Varela MC, Simoes-Sato AY, Kim CA, Bertola DR, De Castro CI, Koiffmann CP . A new case of interstitial 6q16.2 deletion in a patient with Prader-Willi-like phenotype and investigation of SIM1 gene deletion in 87 patients with syndromic obesity. Eur J Med Genet 2006; 49: 298–305.
Bonaglia MC, Ciccone R, Gimelli G, Gimelli S, Marelli S, Verheij J et al. Detailed phenotype-genotype study in five patients with chromosome 6q16 deletion: narrowing the critical region for Prader-Willi-like phenotype. Eur J Hum Genet 2008; 16: 1443–1449.
Stutzmann F, Ghoussaini M, Couturier C, Marchand M, Vatin V, Corset L et al. Loss-of-function mutations in SIM1 cause a specific form of Prader-Willi-like syndrome. Diabetologia 2009; 52 (Suppl 1): 104.
Bonnefond A, Raimondo A, Stutzmann F, Ghoussaini M, Ramachandrappa S, Bersten DC et al. Loss-of-function mutations in SIM1 contribute to obesity and Prader-Willi-like features. J Clin Invest 2013; 123: 3037–3041.
Ramachandrappa S, Raimondo A, Cali AM, Keogh JM, Henning E, Saeed S et al. Rare variants in single-minded 1 (SIM1) are associated with severe obesity. J Clin Invest 2013; 123: 3042–3050.
Roelants M, Hauspie R, Hoppenbrouwers K . References for growth and pubertal development from birth to 21 years in Flanders, Belgium. Ann Hum Biol 2009; 36: 680–694.
Miller SA, Dykes DD, Polesky HF . A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988; 16: 1215.
Wittwer CT, Reed GH, Gundry CN, Vandersteen JG, Pryor RJ . High-resolution genotyping by amplicon melting analysis using LCGreen. Clin Chem 2003; 49: 853–860.
Zhou L, Wang L, Palais R, Pryor R, Wittwer CT . High-resolution DNA melting analysis for simultaneous mutation scanning and genotyping in solution. Clin Chem 2005; 51: 1770–1777.
Zegers D, Beckers S, de Freitas F, Peeters AV, Mertens IL, Verhulst SL et al. Identification of three novel genetic variants in the melanocortin-3 receptor of obese children. Obesity 2011; 19: 152–159.
Ramensky V, Bork P, Sunyaev S . Human non-synonymous SNPs: server and survey. Nucleic Acids Res 2002; 30: 3894–3900.
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P et al. A method and server for predicting damaging missense mutations. Nat Methods 2010; 7: 248–249.
Ng PC, Henikoff S . Predicting deleterious amino acid substitutions. Genome Res 2001; 11: 863–874.
Calabrese R, Capriotti E, Fariselli P, Martelli PL, Casadio R . Functional annotations improve the predictive score of human disease-related mutations in proteins. Hum Mutat 2009; 30: 1237–1244.
Li B, Krishnan VG, Mort ME, Xin F, Kamati KK, Cooper DN et al. Automated inference of molecular mechanisms of disease from amino acid substitutions. Bioinformatics 2009; 25: 2744–2750.
1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature 2010; 467: 1061–1073.
Exome Variant Server, NHLBI Exome Sequencing Project (ESP), Seattle, WA [database on the Internet] 2012. [cited July 14]. Available from: URL http://evs.gs.washington.edu/EVS/.
Wang JC, Turner L, Lomax B, Eydoux P . A 5-Mb microdeletion at 6q16.1-q16.3 with SIM gene deletion and obesity. Am J Med Genet A 2008; 146A: 2975–2978.
Swarbrick MM, Evans DS, Valle MI, Favre H, Wu SH, Njajou OT et al. Replication and extension of association between common genetic variants in SIM1 and human adiposity. Obesity 2011; 19: 2394–2403.
Hung CC, Luan J, Sims M, Keogh JM, Hall C, Wareham NJ et al. Studies of the SIM1 gene in relation to human obesity and obesity-related traits. Int J Obes 2007; 31: 429–434.
Ghoussaini M, Stutzmann F, Couturier C, Vatin V, Durand E, Lecoeur C et al. Analysis of the SIM1 contribution to polygenic obesity in the French population. Obesity 2010; 18: 1670–1675.
Traurig M, Mack J, Hanson RL, Ghoussaini M, Meyre D, Knowler WC et al. Common variation in SIM1 is reproducibly associated with BMI in Pima Indians. Diabetes 2009; 58: 1682–1689.
Crews ST . PAS Proteins: Regulators and Sensors of Development and Physiology 1 edn Kluwer Academic Publishers, 2003.
Chrast R, Scott HS, Chen H, Kudoh J, Rossier C, Minoshima S et al. Cloning of two human homologs of the Drosophila single-minded gene SIM1 on chromosome 6q and SIM2 on 21q within the Down syndrome chromosomal region. Genome Res 1997; 7: 615–624.
Acknowledgements
Research was funded by a PhD grant of the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen) to DZ. SB is a postdoctoral fellow of the Research Foundation—Flanders (FWO Vlaanderen). This work was supported by a grant (G0028.05) from the Research Foundation—Flanders (FWO Vlaanderen) to LVG and WVH and by a TOP-research grant from the University of Antwerp to WVH. This study was supported by an Interuniversity Attraction Pole Project (Phase VII project 43, BELSPO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zegers, D., Beckers, S., Hendrickx, R. et al. Mutation screen of the SIM1 gene in pediatric patients with early-onset obesity. Int J Obes 38, 1000–1004 (2014). https://doi.org/10.1038/ijo.2013.188
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2013.188
Keywords
This article is cited by
-
A Comprehensive Review of Syndromic Forms of Obesity: Genetic Etiology, Clinical Features and Molecular Diagnosis
Current Obesity Reports (2024)
-
Epigenome-wide association study in Chinese monozygotic twins identifies DNA methylation loci associated with blood pressure
Clinical Epigenetics (2023)
-
Cryptic breakpoint identified by whole-genome mate-pair sequencing in a rare paternally inherited complex chromosomal rearrangement
Molecular Cytogenetics (2018)
-
Incomplete penetrance and phenotypic variability of 6q16 deletions including SIM1
European Journal of Human Genetics (2015)