Abstract
Objective:
To investigate the inhibitory effect of cocoa polyphenol extract (CPE) on adipogenesis and obesity along with its mechanism of action.
Methods and Results:
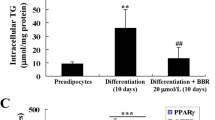
3T3-L1 preadipocytes were cultured with isobutylmethylxanthine, dexamethasone and insulin (MDI), and male C57BL/6N mice (N=44) were fed a high-fat diet (HFD) for 5 weeks with or without CPE. CPE at 100 or 200 μg ml−1 inhibited MDI-induced lipid accumulation without diminishing cell viability. In particular, CPE reduced the protein expression levels of PPARγ and CEBPα, and blocked mitotic clonal expansion (MCE) of preadipocytes by reducing proliferating signaling pathways. This in turn attenuates lipid accumulation during the differentiation of 3T3-L1 preadipocytes. CPE effectively suppressed MDI-induced phosphorylation of extracellular signal-regulated kinase (ERK) and Akt, and their downstream signals. We then examined whether CPE regulates insulin receptor (IR), a common upstream regulator of ERK and Akt. We found that although CPE does not affect the protein expression level of IR, it significantly inhibits the activity of IR kinase via direct binding. Collectively, the results suggested that CPE, a direct inhibitor of IR kinase activity, inhibits cellular differentiation and lipid accumulation in 3T3-L1 preadipocytes. Consistently, CPE attenuated HFD-induced body weight gain and fat accumulation in obese mice fed with a HFD. We also found that HFD-induced increased fasting glucose levels remained unaffected by CPE.
Conclusion:
This study demonstrates that CPE inhibits IR kinase activity and its proliferative downstream signaling markers, such as ERK and Akt, in 3T3-L1 preadipocytes, and also prevents the development of obesity in mice fed with a HFD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hotamisligil G . Inflammation and metabolic disorders. Nature 2006; 444: 860–867.
Kopelman P . Obesity as a medical problem. Nature 2000; 404: 635–643.
Shepherd PR, Gnudi L, Tozzo E, Yang H, Leach F, Kahn BB . Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J Biol Chem 1993; 268: 22243–22246.
Naaz A, Holsberger D, Iwamoto G, Nelson A, Kiyokawa H, Cooke P . Loss of cyclin-dependent kinase inhibitors produces adipocyte hyperplasia and obesity. FASEB J 2004; 18: 1925–1927.
Lobstein T, Baur L, Uauy R . Obesity in children and young people: a crisis in public health. Obes Rev 2004; 5 (s1): 4–85.
Macdougald OA, Lane MD . Transcriptional regulation of gene-expression during adipocyte differentiation. Annu Rev Biochem 1995; 64: 345–373.
Lane MD, Tang QQ . From multipotent stem cell to adipocyte. Birth Defects Res 2005; 73: 476–477.
Tang Q-Q, Otto TC, Lane MD . Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci USA 2003; 100: 44–49.
Boulton TG, Nye SH, Robbins DJ, Ip NY, Radzlejewska E, Morgenbesser SD et al. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell 1991; 65: 663–675.
Prusty D, Park B, Davis K, Farmer S . Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor γ (PPARγ) and C/EBPα gene expression during the differentiation of 3T3-L1 preadipocytes. J Biol Chem 2002; 277: 46226.
Bost F, Aouadi M, Caron L, Binetruy B . The role of MAPKs in adipocyte differentiation and obesity. Biochimie 2005; 87: 51–56.
Taniguchi C, Emanuelli B, Kahn C . Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 2006; 7: 85–96.
Tang QQ, Gronborg M, Huang H, Kim JW, Otto TC, Pandey A et al. Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis. Proc Natl Acad Sci USA 2005; 102: 9766–9771.
Miyaoka Y, Tanaka M, Naiki T, Miyajima A . Oncostatin M inhibits adipogenesis through the RAS/ERK and STAT5 signaling pathways. J Biol Chem 2006; 281: 37913–37920.
Li X, Kim JW, Gronborg M, Urlaub H, Lane MD, Tang QQ . Role of cdk2 in the sequential phosphorylation/activation of C/EBPbeta during adipocyte differentiation. Proc Natl Acad Sci USA 2007; 104: 11597–11602.
Xu J, Liao K . Protein kinase B/AKT 1 plays a pivotal role in insulin-like growth factor-1 receptor signaling induced 3T3-L1 adipocyte differentiation. J Biol Chem 2004; 279: 35914.
Hu E, Kim J, Sarraf P, Bruce M . Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science 1996; 274: 2100.
White MF, Shoelson SE, Keutmann H, Kahn CR . A cascade of tyrosine autophosphorylation in the beta-subunit activates the phosphotransferase of the insulin receptor. J Biol Chem 1988; 263: 2969–2980.
Saltiel AR, Kahn CR . Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001; 414: 799–806.
Miki H, Yamauchi T, Suzuki R, Komeda K, Tsuchida A, Kubota N et al. Essential role of insulin receptor substrate 1 (IRS-1) and IRS-2 in adipocyte differentiation. Mol Cell Biol 2001; 21: 2521.
Bluher M, Michael M, Peroni O, Ueki K, Carter N, Kahn B et al. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell 2002; 3: 25–38.
Lee K, Kim Y, Lee H, Lee C . Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J Agric Food Chem 2003; 51: 7292–7295.
Ruzaidi A, Amin I, Nawalyah A, Hamid M, Faizul H . The effect of Malaysian cocoa extract on glucose levels and lipid profiles in diabetic rats. J Ethnopharmacol 2005; 98: 55–60.
Lamuela-Raventos R, Romero-Perez A, Andres-Lacueva C, Tornero A Health effects of cocoa flavonoids. Food Sci Technol Int 2005; 11: 159.
Taubert D, Roesen R, Lehmann C, Jung N, Schomig E . Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA 2007; 298: 49.
Kang N, Lee K, Lee D, Rogozin E, Bode A, Lee H et al. Cocoa procyanidins suppress transformation by inhibiting mitogen-activated protein kinase kinase. J Biol Chem 2008; 283: 20664.
Balzer J, Rassaf T, Heiss C, Kleinbongard P, Lauer T, Merx M et al. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients: a double-masked, randomized, controlled trial. J Am Coll Cardiol 2008; 51: 2141.
Matsui N, Ito R, Nishimura E, Yoshikawa M, Kato M, Kamei M et al. Ingested cocoa can prevent high-fat diet-induced obesity by regulating the expression of genes for fatty acid metabolism. Nutrition 2005; 21: 594–601.
Lee K, Kundu J, Kim S, Chun K, Lee H, Surh Y . Cocoa polyphenols inhibit phorbol ester-Induced superoxide anion formation in cultured HL-60 cells and expression of cyclooxygenase-2 and activation of NF-{kappa} B and MAPKs in mouse skin in vivo. J Nutr 2006; 136: 1150.
Cho ES, Jang YJ, Kang NJ, Hwang MK, Kim YT, Lee KW et al. Cocoa procyanidins attenuate 4-hydroxynonenal-induced apoptosis of PC12 cells by directly inhibiting mitogen-activated protein kinase kinase 4 activity. Free Radic Biol Med 2009; 46: 1319–1327.
Cristancho AG, Lazar MA . Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol 2011; 12: 722–734.
Zhang W, Liu HT . MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 2002; 12: 9–18.
Manning BD, Cantley LC . AKT/PKB signaling: navigating downstream. Cell 2007; 129: 1261–1274.
Avruch J . Insulin signal transduction through protein kinase cascades. Mol Cell Biochem 1998; 182: 31–48.
Taylor S . Lilly Lecture: molecular mechanisms of insulin resistance. Lessons from patients with mutations in the insulin-receptor gene. Diabetes 1992; 41: 1473.
Arner P, Pollare T, Lithell H, Livingston J . Defective insulin receptor tyrosine kinase in human skeletal muscle in obesity and type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1987; 30: 437–440.
Michael M, Kulkarni R, Postic C, Previs S, Shulman G, Magnuson M et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell 2000; 6: 87–97.
Grassi D, Necozione S, Lippi C, Croce G, Valeri L, Pasqualetti P et al. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension 2005; 46: 398–405.
Bruning J, Michael M, Winnay J, Hayashi T, Horsch D, Accili D et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell 1998; 2: 559–569.
Zick Y . Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Science’s STKE 2005; 2005: pe4.
Bouzakri K, Roques M, Gual P, Espinosa S, Guebre-Egziabher F, Riou J et al. Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes 2003; 52: 1319.
Harrington L, Findlay G, Gray A, Tolkacheva T, Wigfield S, Rebholz H et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol 2004; 166: 213.
Miller B, Shankavaram U, Horney M, Gore A, Kurtz D, Rosenzweig S . Activation of cJun NH2-terminal kinase/stress-activated protein kinase by insulin†. Biochemistry 1996; 35: 8769–8775.
Hong S, Huo H, Xu J, Liao K . Insulin-like growth factor-1 receptor signaling in 3T3-L1 adipocyte differentiation requires lipid rafts but not caveolae. Cell Death Differ 2004; 11: 714–723.
de la Garza AL, Milagro FI, Boque N, Campion J, Martinez JA . Natural inhibitors of pancreatic lipase as new players in obesity treatment. Planta Medica 2011; 77: 773–785.
Tanaka T, Matsuo Y, Kouno I . Chemistry of secondary polyphenols produced during processing of tea and selected foods. Int J Mol Sci 2009; 11: 14–40.
Gu Y, Hurst WJ, Stuart DA, Lambert JD . Inhibition of key digestive enzymes by cocoa extracts and procyanidins. J Agric Food Chem 2011; 59: 5305–5311.
Acknowledgements
This work was supported by grants from the World Class University Program (R31-2008-00-10056-0) and the National Leap Research Program (No. 2010-0029233) through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology, Republic of Korea.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Min, S., Yang, H., Seo, S. et al. Cocoa polyphenols suppress adipogenesis in vitro and obesity in vivo by targeting insulin receptor. Int J Obes 37, 584–592 (2013). https://doi.org/10.1038/ijo.2012.85
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2012.85
Keywords
This article is cited by
-
The effect of high-polyphenol Mediterranean diet on visceral adiposity: the DIRECT PLUS randomized controlled trial
BMC Medicine (2022)
-
Nonerythropoietic Erythropoietin-Derived Peptide Suppresses Adipogenesis, Inflammation, Obesity and Insulin Resistance
Scientific Reports (2015)
-
Persimmon tannin represses 3T3-L1 preadipocyte differentiation via up-regulating expression of miR-27 and down-regulating expression of peroxisome proliferator-activated receptor-γ in the early phase of adipogenesis
European Journal of Nutrition (2015)
-
Antioxidant and anti-adipogenic activities of persimmon tannins
Food Science and Biotechnology (2014)