Abstract
Background:
Bisphenol A (BPA) is considered as an environmental obesogen. The enzyme 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) converts the inactive hormone cortisone to the active hormone cortisol in adipose tissues and promotes adipogenesis.
Objective:
To examine whether environmentally relevant concentrations of BPA could increase the expression of 11β-HSD1, as well as that of the adipogenesis-related genes peroxisome proliferator-activated receptor-γ (PPAR-γ) and lipoprotein lipase (LPL), in the adipose tissue of children.
Methods:
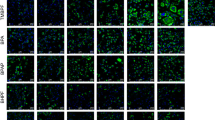
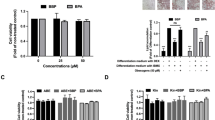
Omental fat biopsies were obtained from 17 children (7 boys and 10 girls between 3 and 13 years of age) undergoing abdominal surgery. The effects of BPA (10 nM, 1 μM, and 80 μM) on 11β-HSD1, PPAR-γ and LPL mRNA expression, and 11β-HSD1 enzymatic activity in adipose tissue and adipocytes were assessed in vitro. Moreover, the effects of carbenoxolone (CBX), an 11β-HSD1 inhibitor, or RU486, a glucocorticoid (GC) receptor antagonist, on 11β-HSD1, PPAR-γ and LPL mRNA expression were assessed in human visceral preadipocytes and adipocytes.
Results:
BPA, even at the lowest concentration tested (10 nM), increased the mRNA expression and enzymatic activity of 11β-HSD1 in the omental adipose tissue samples and the visceral adipocytes. Similar effects on PPAR-γ and LPL mRNA expression and lipid accumulation were observed in the adipocytes. CBX treatment inhibited the stimulatory effects of BPA (at 10 nM) on PPAR-γ and LPL mRNA expression, whereas RU486 inhibited 11β-HSD1 mRNA expression in the adipocytes.
Conclusion:
BPA, at environmentally relevant levels, increased the mRNA expression and enzymatic activity of 11β-HSD1 by acting upon a GC receptor, which may lead to the acceleration of adipogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Baillie-Hamilton PF . Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med 2002; 8: 185–192.
Rubin BS . Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol 2011; 127: 27–34.
Nam SH, Seo YM, Kim MG . Bisphenol A migration from polycarbonate baby bottle with repeated use. Chemosphere 2010; 79: 949–952.
Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G . Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 2010; 118: 1055–1070.
Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV . Human exposure to bisphenol A (BPA). Reprod Toxicol 2007; 24: 139–177.
Welshons WV, Nagel SC, Vom SF . Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 2006; 147: S56–S69.
Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL . Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect 2008; 116: 39–44.
Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, Vom SF . Exposure to bisphenol A advances puberty. Nature 1999; 401: 763–764.
Rubin BS, Murray MK, Damassa DA, King JC, Soto AM . Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect 2001; 109: 675–680.
Somm E, Schwitzgebel VM, Toulotte A, Cederroth CR, Combescure C, Nef S et al. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ Health Perspect 2009; 117: 1549–1555.
Masuno H, Kidani T, Sekiya K, Sakayama K, Shiosaka T, Yamamoto H et al. Bisphenol A in combination with insulin can accelerate the conversion of 3T3-L1 fibroblasts to adipocytes. J Lipid Res 2002; 43: 676–684.
Masuno H, Iwanami J, Kidani T, Sakayama K, Honda K . Bisphenol a accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicol Sci 2005; 84: 319–327.
Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N . Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect 2008; 116: 1642–1647.
Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR et al. A transgenic model of visceral obesity and the metabolic syndrome. Science 2001; 294: 2166–2170.
Tomlinson JW, Sherlock M, Hughes B, Hughes SV, Kilvington F, Bartlett W et al. Inhibition of 11Beta-hydroxysteroid dehydrogenase type 1 activity in vivo limits glucocorticoid exposure to human adipose tissue and decreases lipolysis. J Clin Endocrinol Metab 2007; 92: 857–864.
Morton NM, Seckl JR . 11Beta-hydroxysteroid dehydrogenase type 1 and obesity. Front Horm Res 2008; 36: 146–164.
Rask E, Walker BR, Soderberg S, Livingstone DE, Eliasson M, Johnson O et al. Tissue-specific changes in peripheral cortisol metabolism in obese women: increased adipose 11beta-hydroxysteroid dehydrogenase type 1 activity. J Clin Endocrinol Metab 2002; 87: 3330–3336.
Munoz R, Carvajal C, Escalona A, Boza C, Perez G, Ibanez L et al. 11Beta-hydroxysteroid dehydrogenase type 1 is overexpressed in subcutaneous adipose tissue of morbidly obese patients. Obes Surg 2009; 19: 764–770.
Li X, Lindquist S, Chen R, Myrnas T, Angsten G, Olsson T et al. Depot-specific messenger RNA expression of 11 beta-hydroxysteroid dehydrogenase type 1 and leptin in adipose tissue of children and adults. Int J Obes (Lond) 2007; 31: 820–828.
Wake DJ, Walker BR . Inhibition of 11beta-hydroxysteroid dehydrogenase type 1 in obesity. Endocrine 2006; 29: 101–108.
Zhu L, Hou M, Sun B, Buren J, Zhang L, Yi J et al. Testosterone stimulates adipose tissue 11beta-hydroxysteroid dehydrogenase type 1 expression in a depot-specific manner in children. J Clin Endocrinol Metab 2010; 95: 3300–3308.
Bujalska IJ, Quinkler M, Tomlinson JW, Montague CT, Smith DM, Stewart PM . Expression profiling of 11beta-hydroxysteroid dehydrogenase type-1 and glucocorticoid-target genes in subcutaneous and omental human preadipocytes. J Mol Endocrinol 2006; 37: 327–340.
Spencer SJ, Tilbrook A . The glucocorticoid contribution to obesity. Stress 2011; 14: 233–246.
Fredriks AM, van Buuren S, Wit JM, Verloove-Vanhorick SP . Body index measurements in 1996-7 compared with 1980. Arch Dis Child 2000; 82: 107–112.
Gesta S, Lolmede K, Daviaud D, Berlan M, Bouloumie A, Lafontan M et al. Culture of human adipose tissue explants leads to profound alteration of adipocyte gene expression. Horm Metab Res 2003; 35: 158–163.
Vankoningsloo S, Piens M, Lecocq C, Gilson A, De Pauw A, Renard P et al. Mitochondrial dysfunction induces triglyceride accumulation in 3T3-L1 cells: role of fatty acid beta-oxidation and glucose. J Lipid Res 2005; 46: 1133–1149.
Pfaffl MW . A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29: e45.
Monder C, Lakshmi V, Miroff Y . Kinetic studies on rat liver 11 beta-hydroxysteroid dehydrogenase. Biochim Biophys Acta 1991; 1115: 23–29.
Walker EA, Clark AM, Hewison M, Ride JP, Stewart PM . Functional expression, characterization, and purification of the catalytic domain of human 11-beta -hydroxysteroid dehydrogenase type 1. J Biol Chem 2001; 276: 21343–21350.
Sargis RM, Johnson DN, Choudhury RA, Brady MJ . Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity (Silver Spring) 2010; 18: 1283–1288.
Sakurai K, Kawazuma M, Adachi T, Harigaya T, Saito Y, Hashimoto N et al. Bisphenol A affects glucose transport in mouse 3T3-F442A adipocytes. Br J Pharmacol 2004; 141: 209–214.
Epel ES, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD et al. Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom Med 2000; 62: 623–632.
Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, Cooper MS et al. 11Beta-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev 2004; 25: 831–866.
Hutley LJ, Newell FM, Joyner JM, Suchting SJ, Herington AC, Cameron DP et al. Effects of rosiglitazone and linoleic acid on human preadipocyte differentiation. Eur J Clin Invest 2003; 33: 574–581.
Laplante M, Sell H, MacNaul KL, Richard D, Berger JP, Deshaies Y . PPAR-gamma activation mediates adipose depot-specific effects on gene expression and lipoprotein lipase activity: mechanisms for modulation of postprandial lipemia and differential adipose accretion. Diabetes 2003; 52: 291–299.
Pantoja C, Huff JT, Yamamoto KR . Glucocorticoid signaling defines a novel commitment state during adipogenesis in vitro. Mol Biol Cell 2008; 19: 4032–4041.
Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998; 139: 4252–4263.
Gumy C, Chandsawangbhuwana C, Dzyakanchuk AA, Kratschmar DV, Baker ME, Odermatt A . Dibutyltin disrupts glucocorticoid receptor function and impairs glucocorticoid-induced suppression of cytokine production. PLoS One 2008; 3: e3545.
Johansson M, Johansson N, Lund BO . Xenobiotics and the glucocorticoid receptor: additive antagonistic effects on tyrosine aminotransferase activity in rat hepatoma cells. Basic Clin Pharmacol Toxicol 2005; 96: 309–315.
Diederich S, Grossmann C, Hanke B, Quinkler M, Herrmann M, Bahr V et al. In the search for specific inhibitors of human 11beta-hydroxysteroid-dehydrogenases (11beta-HSDs): chenodeoxycholic acid selectively inhibits 11beta-HSD-I. Eur J Endocrinol 2000; 142: 200–207.
Stewart PM . 11 Beta-hydroxysteroid dehydrogenase: implications for clinical medicine. Clin Endocrinol (Oxf) 1996; 44: 493–499.
Acknowledgements
This work was supported through funding from 973 Program of China (2013CB530604), the National Natural Science Foundation of China (81273064), the Blue Project of the Jiangsu Education Department of China (JX10410533), the Scientific Research Foundation for Returned Overseas Scholars of the Ministry of Education of China (DG216G15013), and the Project Funder by the Priority Academic Program Development of Jiangsu Higher Education Institutions. We thank the doctors in the Department of Surgery at Nanjing Children’s Hospital for collecting samples, Associate Professor Baoqing Mo (Department of Public Health, Nanjing Medical University) for help with statistical analysis, and Professor Duan Chen (Department of Cancer Research and Molecular Medicine, Norwegian University of Science and Technology) for valuable discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, J., Sun, B., Hou, M. et al. The environmental obesogen bisphenol A promotes adipogenesis by increasing the amount of 11β-hydroxysteroid dehydrogenase type 1 in the adipose tissue of children. Int J Obes 37, 999–1005 (2013). https://doi.org/10.1038/ijo.2012.173
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2012.173
Keywords
This article is cited by
-
Bisphenol A in fish, seawater, and fishermen’s urine: measurement and health risk assessment in southern Iran
International Journal of Environmental Science and Technology (2023)
-
Implications of endocrine-disrupting chemicals on polycystic ovarian syndrome: A comprehensive review
Environmental Science and Pollution Research (2022)
-
Gestational bisphenol A exposure induces fatty liver development in male offspring mice through the inhibition of HNF1b and upregulation of PPARγ
Cell Biology and Toxicology (2021)
-
Environmental neglect: endocrine disruptors as underappreciated but potentially modifiable diabetes risk factors
Diabetologia (2019)
-
Prenatal bisphenol a exposure and dysregulation of infant hypothalamic-pituitary-adrenal axis function: findings from the APrON cohort study
Environmental Health (2017)