Abstract
Background:
Brown adipose tissue (BAT) is involved in the regulation of whole-body energy expenditure and adiposity. The activity and prevalence of BAT decrease with age in humans.
Objective:
To examine the effects of single nucleotide polymorphisms of the genes for uncoupling protein 1 (UCP1) and β3-adrenergic receptor (β3AR), key molecules of BAT thermogenesis, on age-related decline of BAT activity and accumulation of body fat in humans.
Methods:
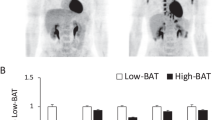
One hundred ninety-nine healthy volunteers (20–72 years old (y.o.)) underwent fluorodeoxyglucose-positron emission tomography (FDG-PET) and computed tomography (CT) after 2-h cold exposure to assess BAT activity. The visceral and subcutaneous fat areas at the abdominal level were estimated from the CT images. They were genotyped for −3826 A/G polymorphism of the UCP1 gene and 64 Trp/Arg mutation of the β3AR gene.
Results:
BAT was detected in 88 subjects out of 199 (44%), more in younger (⩽30 y.o., 55%) than older subjects (>40 y.o., 15%). BAT prevalence of older subjects tended to be lower in the UCP1 G/G group than the A allele group (A/A and A/G), and also in the β3AR Arg allele group (Trp/Arg and Arg/Arg) than the Trp/Trp group. When compared subjects who had two or more base substitutions on the two genes (the 2–4 allele group) with those who had less than two base substitutions (the 0–1 allele group), BAT prevalence was comparable in younger subjects (62% vs 50%) but lower in older subjects (0% vs 24%, P<0.05). Visceral fat area of the 2–4 allele group was higher than that of the 0–1 allele group (P<0.05) in older subjects, but not in younger subjects.
Conclusion:
UCP1 −3826 A/G and β3AR 64 Trp/Arg substitutions accelerate age-related decrease in BAT activity, and thereby may associate with visceral fat accumulation with age.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Cannon B, Nedergaard J . Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84: 277–359.
Lowell BB, Spiegelman BM . Towards a molecular understanding of adaptive thermogenesis. Nature 2000; 404: 652–660.
Cohade C, Osman M, Pannu HK, Wahl RL . Uptake in supraclavicular area fat (‘USA-Fat’): description on 18F-FDG PET/CT. J Nucl Med 2003; 44: 170–176.
Nedergaard J, Bengtsson T, Cannon B . Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2007; 293: E444–E452.
Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009; 360: 1518–1525.
Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J 2009; 23: 3113–3120.
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360: 1509–1517.
Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009; 58: 1526–1531.
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009; 360: 1500–1508.
Yoneshiro T, Aita S, Matsushita M, Kameya T, Nakada K, Kawai Y et al. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity 2011; 19: 13–16.
Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab 2011; 14: 272–279.
Yoneshiro T, Aita S, Matsushita M, Okamatsu-Ogura Y, Kameya T, Kawai Y et al. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity 2011; 19: 1755–1760.
Collins S, Yehuda-Shnaidman E, Wang H . Positive and negative control of ucp1 gene transcription and the role of β-adrenergic signaling networks. Int J Obes 2010; 34: S28–S33.
Oppert JM, Vohl MC, Chagnon M, Dionne FT, Cassard-Doulcier AM, Ricquier D et al. DNA polymorphism in the uncoupling protein (UCP) gene and human body fat. Int J Obes 1994; 18: 526–531.
Jia JJ, Tian YB, Cao ZH, Tao LL, Zhang X, Gao SZ et al. The polymorphisms of UCP1 genes associated with fat metabolism, obesity and diabetes. Mol Biol Rep 2010; 37: 1513–1522.
Del Mar Gonzalez-Barroso M, Ricquier D, Cassard-Doulcier AM . The human uncoupling protein-1 gene (UCP1): present status and perspectives in obesity research. Obes Rev 2000; 1: 61–72.
Walston J, Silver K, Bogardus C, Knowler WC, Celi FS, Austin S et al. Time of onset of non-insulin-dependent diabetes mellitus and genetic variation in the beta 3-adrenergic-receptor gene. N Engl J Med 1995; 333: 343–347.
Arner P, Hoffstedt J . Adrenoceptor genes in human obesity. J Intern Med 1999; 245: 667–672.
Nagai N, Sakane N, Tsuzaki K, Moritani T . UCP1 genetic polymorphism (–3826 A/G) diminishes resting energy expenditure and thermoregulatory sympathetic nervous system activity in young females. Int J Obes 2011; 35: 1050–1055.
Valve R, Heikkinen S, Rissanen A, Laakso M, Uusitupa M . Synergistic effect of polymorphisms in uncoupling protein 1 and β3-adrenergic receptor genes on basal metabolic rate in obese Finns. Diabetologia 1998; 41: 357–361.
Nagai N, Sakane N, Fujishita A, Fujiwara R, Kimura T, Kotani K et al. The −3826 A→G variant of the uncoupling protein-1 gene diminishes thermogenesis during acute cold exposure in healthy children. Obes Res Clin Pract 2007; 1: 99–107.
Nagai N, Sakane N, Ueno LM, Hamada T, Moritani T . The −3826 A→G variant of the uncoupling protein-1 gene diminishes postprandial thermogenesis after a high fat meal in healthy boys. J Clin Endocrinol Metab 2003; 88: 5661–5667.
Kogure A, Yoshida T, Sakane N, Umekawa T, Takakura Y, Kondo M . Synergic effect of polymorphisms in uncoupling protein 1 and beta3-adrenergic receptor genes on weight loss in obese Japanese. Diabetologia 1998; 41: 1399.
Yoshida T, Sakane N, Umekawa T, Sakai M, Takahashi T, Kondo M . Mutation of beta 3-adrenergic-receptor gene and response to treatment of obesity. Lancet 1995; 25: 1433–1434.
van der Kooy K, Seidell JC . Techniques for the measurement of visceral fat: a practical guide. Int J Obes 1993; 17: 187–196.
Rose G, Crocco P, D’Aquila P, Montesanto A, Bellizzi D, Passarino G . Two variants located in the upstream enhancer region of human UCP1 gene affect gene expression and are correlated with human longevity. Exp Gerontol 2011; 46: 897–904.
Esterbauer H, Oberkofler H, Liu YM, Breban D, Hell E, Krempler F et al. Uncoupling protein-1 mRNA expression in obese human subjects: the role of sequence variations at the uncoupling protein-1 gene locus. J Lip Res 1998; 39: 834–844.
Lee P, Zhao JT, Swarbrick MM, Gracie G, Bova R, Greenfield JR et al. High prevalence of brown adipose tissue in adult humans. J Clin Endocrinol Metab 2011; 96: 2450–2455.
Florez-Duquet M, McDonald RB . Cold-induced thermoregulation and biological aging. Physiol Rev 1998; 78: 339–358.
Piétri-Rouxel F, John Manning B, Gros J, Strosberg AD . The biochemical effect of the naturally occurring Trp64→Arg mutation on human beta3-adrenoceptor activity. Eur J Biochem 1997; 247: 1174–1179.
Heilbronn LK, Kind KL, Pancewicz E, Morris AM, Noakes M, Clifton PM . Association of −3826 G variant in uncoupling protein-1 with increased BMI in overweight Australian women. Diabetologia 2000; 43: 242–244.
Hayakawa T, Nagai Y, Taniguchi M, Yamashita H, Takamura T, Abe T et al. Phenotypic characterization of the beta3-adrenergic receptor mutation and the uncoupling protein 1 polymorphism in Japanese men. Metabolism 1999; 48: 636–640.
Urhammer SA, Hansen T, Borch-Johnsen K, Pedersen O . Studies of the synergistic effect of the Trp/Arg64 polymorphism of the beta3-adrenergic receptor gene and the −3826 A→G variant of the uncoupling protein-1 gene on features of obesity and insulin resistance in a population-based sample of 379 young Danish subjects. J Clin Endocrinol Metab 2000; 85: 3151–3154.
Shihara N, Yasuda K, Moritani T, Ue H, Uno M, Adachi T et al. Synergistic effect of polymorphisms of uncoupling protein 1 and beta3-adrenergic receptor genes on autonomic nervous system activity. Int J Obes 2001; 25: 761–766.
Kurokawa N, Young EH, Oka Y, Satoh H, Wareham NJ, Sandhu MS et al. The ADRB3 Trp64Arg variant and BMI: a meta-analysis of 44 833 individuals. Int J Obes 2008; 32: 1240–1249.
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (22590227), and a Special Research Grant from Tenshi College. We thank all subjects for their cooperation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Yoneshiro, T., Ogawa, T., Okamoto, N. et al. Impact of UCP1 and β3AR gene polymorphisms on age-related changes in brown adipose tissue and adiposity in humans. Int J Obes 37, 993–998 (2013). https://doi.org/10.1038/ijo.2012.161
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2012.161
Keywords
This article is cited by
-
Understanding the contemporary high obesity rate from an evolutionary genetic perspective
Hereditas (2023)
-
SIRT7 suppresses energy expenditure and thermogenesis by regulating brown adipose tissue functions in mice
Nature Communications (2022)
-
Adipose tissue aging: mechanisms and therapeutic implications
Cell Death & Disease (2022)
-
Upregulation of cathepsin L gene under mild cold conditions in young Japanese male adults
Journal of Physiological Anthropology (2021)
-
The cellular and functional complexity of thermogenic fat
Nature Reviews Molecular Cell Biology (2021)