Abstract
Objective:
It has not been solved whether subjects carrying the minor alleles of the −455T>C or −482C>T single nucleotide polymorphisms (SNPs) in the apolipoprotein-C3-gene (APOC3) have an increased risk for developing fatty liver and insulin resistance. We investigated the relationships of the SNPs with hepatic APOC3 expression and hypothesized that visceral obesity may modulate the effects of these SNPs on liver fat and insulin sensitivity (IS).
Methods:
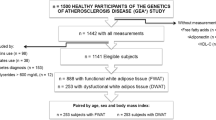
APOC3 mRNA expression and triglyceride content were determined in liver biopsies from 50 subjects. In a separate group (N=330) liver fat was measured by 1H-magnetic resonance spectroscopy. IS was estimated during an oral glucose tolerance test (OGTT) and the euglycemic, hyperinsulinemic clamp (N=222).
Results:
APOC3 mRNA correlated positively with triglyceride content in liver biopsies (r=0.29, P=0.036). Carriers of the minor alleles (−455C and −482T) tended to have higher hepatic APOC3 mRNA expression (1.80 (0.45–3.56) vs 0.77 (0.40–1.64), P=0.09), but not higher triglyceride content (P=0.76). In 330 subjects the genotype did not correlate with liver fat (P=0.97) or IS (OGTT: P=0.41; clamp: P=0.99). However, a significant interaction of the genotype with waist circumference in determining liver fat was detected (P=0.02) in which minor allele carriers had higher liver fat only in the lowest tertile of waist circumference (P=0.01). In agreement, during a 9-month lifestyle intervention the minor allele carriers of the SNP −482C>T in the lowest tertile also had less decrease in liver fat (P=0.04).
Conclusions:
APOC3 mRNA expression is increased in fatty liver and is regulated by SNPs in APOC3. The impact of the APOC3 SNPs on fatty liver is small and depends on visceral obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Fabbrini E, Sullivan S, Klein S . Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 2010; 51: 679–689.
Tilg H, Hotamisligil GS . Nonalcoholic fatty liver disease: cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology 2006; 131: 934–945.
Roden M . Mechanisms of Disease: hepatic steatosis in type 2 diabetes—pathogenesis and clinical relevance. Nat Clin Pract Endocrinol Metab 2006; 2: 335–348.
Stefan N, Kantartzis K, Haring HU . Causes and metabolic consequences of fatty liver. Endocr Rev 2008; 29: 939–960.
Stepanova M, Rafiq N, Younossi ZM . Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population-based study. Gut 2010; 59: 1410–1415.
Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes 2005; 54: 3541–3546.
Kotronen A, Yki-Jarvinen H . Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol 2008; 28: 27–38.
Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA 2009; 106: 15430–15435.
Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med 2008; 168: 1609–1616.
Angulo P . Nonalcoholic fatty liver disease. N Engl J Med 2002; 346: 1221–1231.
Fassio E, Alvarez E, Dominguez N, Landeira G, Longo C . Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology 2004; 40: 820–826.
Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005; 129: 113–121.
Stefan N, Schick F, Haring HU . Sex hormone-binding globulin and risk of type 2 diabetes. N Engl J Med 2009; 361: 2675–2676.
Weikert C, Stefan N, Schulze MB, Pischon T, Berger K, Joost HG et al. Plasma fetuin-a levels and the risk of myocardial infarction and ischemic stroke. Circulation 2008; 118: 2555–2562.
Kantartzis K, Machann J, Schick F, Fritsche A, Haring HU, Stefan N . The impact of liver fat vs visceral fat in determining categories of prediabetes. Diabetologia 2010; 53: 882–889.
Harrison SA, Day CP . Benefits of lifestyle modification in NAFLD. Gut 2007; 56: 1760–1769.
Kantartzis K, Thamer C, Peter A, Machann J, Schick F, Schraml C et al. High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut 2009; 58: 1281–1288.
Tilg H, Moschen A . Update on nonalcoholic fatty liver disease: genes involved in nonalcoholic fatty liver disease and associated inflammation. Curr Opin Clin Nutr Metab Care 2010; 13: 391–396.
Dongiovanni P, Valenti L, Rametta R, Daly AK, Nobili V, Mozzi E et al. Genetic variants regulating insulin receptor signalling are associated with the severity of liver damage in patients with non-alcoholic fatty liver disease. Gut 2010; 59: 267–273.
Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008; 40: 1461–1465.
Kantartzis K, Peter A, Machicao F, Machann J, Wagner S, Konigsrainer I et al. Dissociation between fatty liver and insulin resistance in humans carrying a variant of the patatin-like phospholipase 3 gene. Diabetes 2009; 58: 2616–2623.
Kotronen A, Johansson LE, Johansson LM, Roos C, Westerbacka J, Hamsten A et al. A common variant in PNPLA3, which encodes adiponutrin, is associated with liver fat content in humans. Diabetologia 2009; 52: 1056–1060.
Aalto-Setala K, Fisher EA, Chen X, Chajek-Shaul T, Hayek T, Zechner R et al. Mechanism of hypertriglyceridemia in human apolipoprotein (apo) CIII transgenic mice. Diminished very low density lipoprotein fractional catabolic rate associated with increased apo CIII and reduced apo E on the particles. J Clin Invest 1992; 90: 1889–1900.
de Silva HV, Lauer SJ, Wang J, Simonet WS, Weisgraber KH, Mahley RW et al. Overexpression of human apolipoprotein C-III in transgenic mice results in an accumulation of apolipoprotein B48 remnants that is corrected by excess apolipoprotein E. J Biol Chem 1994; 269: 2324–2335.
Zheng C, Khoo C, Ikewaki K, Sacks FM . Rapid turnover of apolipoprotein C-III-containing triglyceride-rich lipoproteins contributing to the formation of LDL subfractions. J Lipid Res 2007; 48: 1190–1203.
Olivieri O, Bassi A, Stranieri C, Trabetti E, Martinelli N, Pizzolo F et al. Apolipoprotein C-III, metabolic syndrome, and risk of coronary artery disease. J Lipid Res 2003; 44: 2374–2381.
Li WW, Dammerman MM, Smith JD, Metzger S, Breslow JL, Leff T . Common genetic variation in the promoter of the human apo CIII gene abolishes regulation by insulin and may contribute to hypertriglyceridemia. J Clin Invest 1995; 96: 2601–2605.
Olivieri O, Stranieri C, Bassi A, Zaia B, Girelli D, Pizzolo F et al. ApoC-III gene polymorphisms and risk of coronary artery disease. J Lipid Res 2002; 43: 1450–1457.
Tilly P, Sass C, Vincent-Viry M, Aguillon D, Siest G, Visvikis S . Biological and genetic determinants of serum apoC-III concentration: reference limits from the Stanislas Cohort. J Lipid Res 2003; 44: 430–436.
Ferns GA, Stocks J, Ritchie C, Galton DJ . Genetic polymorphisms of apolipoprotein C-III and insulin in survivors of myocardial infarction. Lancet 1985; 2: 300–303.
Petersen KF, Dufour S, Hariri A, Nelson-Williams C, Foo JN, Zhang XM et al. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. N Engl J Med 2010; 362: 1082–1089.
Kozlitina J, Boerwinkle E, Cohen JC, Hobbs HH . Dissociation between APOC3 variants, hepatic triglyceride content and insulin resistance. Hepatology 2011; 53: 467–474.
Diehl AM . Genetic susceptibility to hepatic steatosis. N Engl J Med 2010; 362: 1142–1143.
Ruiz-Narvaez EA, Sacks FM, Campos H . Abdominal obesity and hyperglycemia mask the effect of a common APOC3 haplotype on the risk of myocardial infarction. Am J Clin Nutr 2008; 87: 1932–1938.
Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F . Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol 2009; 51: 433–445.
Matsuda M, DeFronzo RA . Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22: 1462–1470.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419.
Stefan N, Machicao F, Staiger H, Machann J, Schick F, Tschritter O et al. Polymorphisms in the gene encoding adiponectin receptor 1 are associated with insulin resistance and high liver fat. Diabetologia 2005; 48: 2282–2291.
Stefan N, Fritsche A, Weikert C, Boeing H, Joost HG, Haring HU et al. Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes 2008; 57: 2762–2767.
Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Krober SM et al. Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care 2006; 29: 853–857.
Jong MC, Hofker MH, Havekes LM . Role of ApoCs in lipoprotein metabolism: functional differences between ApoC1, ApoC2, and ApoC3. Arterioscler Thromb Vasc Biol 1999; 19: 472–484.
Ito Y, Azrolan N, O’Connell A, Walsh A, Breslow JL . Hypertriglyceridemia as a result of human apo CIII gene expression in transgenic mice. Science 1990; 249: 790–793.
Maeda N, Li H, Lee D, Oliver P, Quarfordt SH, Osada J . Targeted disruption of the apolipoprotein C-III gene in mice results in hypotriglyceridemia and protection from postprandial hypertriglyceridemia. J Biol Chem 1994; 269: 23610–23616.
von Eckardstein A, Holz H, Sandkamp M, Weng W, Funke H, Assmann G . Apolipoprotein C-III(Lys58----Glu). Identification of an apolipoprotein C-III variant in a family with hyperalphalipoproteinemia. J Clin Invest 1991; 87: 1724–1731.
Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science 2008; 322: 1702–1705.
Kawakami A, Aikawa M, Libby P, Alcaide P, Luscinskas FW, Sacks FM . Apolipoprotein CIII in apolipoprotein B lipoproteins enhances the adhesion of human monocytic cells to endothelial cells. Circulation 2006; 113: 691–700.
Kawakami A, Aikawa M, Nitta N, Yoshida M, Libby P, Sacks FM . Apolipoprotein CIII-induced THP-1 cell adhesion to endothelial cells involves pertussis toxin-sensitive G protein- and protein kinase C alpha-mediated nuclear factor-kappaB activation. Arterioscler Thromb Vasc Biol 2007; 27: 219–225.
Altomonte J, Cong L, Harbaran S, Richter A, Xu J, Meseck M et al. Foxo1 mediates insulin action on apoC-III and triglyceride metabolism. J Clin Invest 2004; 114: 1493–1503.
van Hoek M, van Herpt TW, Dehghan A, Hofman A, Lieverse AG, van Duijn CM, Witteman JC, Sijbrands EJ . Association of an APOC3 promoter variant with type 2 diabetes risk and need for insulin treatment in lean persons. Diabetologia 2011; 54: 1360–1367.
Kantartzis K, Machicao F, Machann J, Schick F, Fritsche A, Haring HU et al. The DGAT2 gene is a candidate for the dissociation between fatty liver and insulin resistance in humans. Clin Sci (Lond) 2009; 116: 531–537.
Amaro A, Fabbrini E, Kars M, Yue P, Schechtman K, Schonfeld G et al. Dissociation between intrahepatic triglyceride content and insulin resistance in familial hypobetalipoproteinemia. Gastroenterology 2010; 139: 149–153.
Acknowledgements
We would like to thank all the participants for their cooperation and Roman Werner, Melanie Weisser, Alke Guirguis and Mareike Walenta and the phenotyping team for their help in collecting the data. The study was supported by grants from the Deutsche Forschungsgemeinschaft (KFO 114 and a Heisenberg-Grant to NS, STE 1096/1-1), the European Community's FP6 EUGENE2 (LSHM-CT-2004-512013) and the German Federal Ministry of Education and Research (DLR01GI0925).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Peter, A., Kantartzis, K., Machicao, F. et al. Visceral obesity modulates the impact of apolipoprotein C3 gene variants on liver fat content. Int J Obes 36, 774–782 (2012). https://doi.org/10.1038/ijo.2011.154
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2011.154
Keywords
This article is cited by
-
Acylated- and unacylated ghrelin during an oral glucose tolerance test in humans at risk for type 2 diabetes mellitus
International Journal of Obesity (2023)
-
Apolipoprotein C-III and its defined lipoprotein subspecies in relation to incident diabetes: the Multi-Ethnic Study of Atherosclerosis
Diabetologia (2019)
-
Associations of the APOB rs693 and rs17240441 polymorphisms with plasma APOB and lipid levels: a meta-analysis
Lipids in Health and Disease (2017)
-
Apolipoprotein A5 and apolipoprotein C3 single nucleotide polymorphisms are correlated with an increased risk of coronary heart disease: a case–control and meta-analysis study
Lipids in Health and Disease (2015)
-
The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes
Nature (2014)