Abstract
Objective:
The aim of this study is to investigate the effect of body size on insulin-mediated, whole-body glucose uptake (M-value) in morbidly obese (MO) subjects, who have large amounts of fat mass. Furthermore, we aimed at verifying which surrogate insulin-sensitivity index can better substitute the euglycemic clamp values and whether the insulin secretion/insulin resistance index is meaningful also in MO subjects.
Design:
The study design is cross-sectional, case–control study of insulin sensitivity—assessed by different methods—and insulin secretion.
Subjects:
One-hundred and sixty-eight subjects ca. 39 years old, with a body mass index (BMI) between 17 and 64 kg m–2, underwent euglycemic hyperinsulinemic clamp and oral glucose tolerance test (OGTT) with surrogate measures of insulin sensitivity together with body composition by 3H2O dilution. Insulin secretion rate (ISR) was measured at fast and after OGTT by C-peptide deconvolution.
Results:
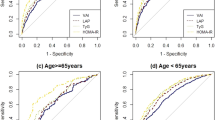
The population was divided into quartiles of BMI. In the fourth quartile, the best insulin-sensitivity variable between M/I/kgFFM and M/I/kgbw was the latter, as shown by area under the receiver–operator characteristic (ROC) curve (0.85 vs 0.89). The best index to identify insulin-resistant individuals (lowest distribution quartile: M/I/kgbw⩽29.3 μmol min−1 kg−1 nmol l−1) were Matsuda index and oral glucose insulin sensitivity (OGIS), whereas fasting insulin concentration, QUICKI, and HOMA failed (ROC analysis). M-value declined exponentially as the BMI increased, whereas ISR linearly increased. The insulin secretion/insulin resistance index well applied to MO.
Conclusion:
In MO subjects, in which the fat mass is highly represented, fat-free mass cannot be considered the only determinant of insulin sensitivity, thus M-value should be normalized by total body weight. The best surrogates of insulin sensitivity measured by euglycemic clamp are Matsuda index and OGIS. BMI directly affects both insulin sensitivity and ISR and the insulin secretion/insulin resistance index is a valid model to correlate ISR with insulin sensitivity also in MO.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Müller MJ, Bosy-Westphal A, Kutzner D, Heller M . Metabolically active components of fat-free mass and resting energy expenditure in humans: recent lessons from imaging technologies. Obes Rev 2002; 3: 113–122.
Van Gaal LF, Vansant GA, De Leeuw IH . Factors determining energy expenditure during very-low-calorie diets. Am J Clin Nutr 1992; 56: 224S–229S.
Luke A, Schoeller DA . Basal metabolic rate, fat-free mass and body cell mass during energy restriction. Metabolism 1992; 41: 450–456.
Gastaldelli A, Cusi K, Pettiti M, Hardies J, Miyazaki Y, Berria R et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 2007; 133: 496–506.
Gastaldelli A, Miyazaki Y, Mahankali A, Berria R, Pettiti M, Buzzigoli E et al. The effect of pioglitazone on the liver: role of adiponectin. Diabetes Care 2006; 29: 2275–2281.
Fernández-Real JM, Straczkowski M, Lainez B, Chacón MR, Kowalska I, López-Bermejo A et al. An alternative spliced variant of circulating soluble tumor necrosis factor-alpha receptor-2 is paradoxically associated with insulin action. Eur J Endocrinol 2006; 154: 723–730.
Balkau B, Mhamdi L, Oppert JM, Nolan J, Golay A, Porcellati F et al. Physical activity and insulin sensitivity: the RISC study. Diabetes 2008; 57: 2613–2618.
Calvani M, Scarfone A, Granato L, Mora EV, Nanni G, Castagneto M et al. Restoration of adiponectin pulsatility in severely obese subjects after weight loss. Diabetes 2004; 53: 939–947.
Mingrone G, Henriksen FL, Greco AV, Krogh LN, Capristo E, Gastaldelli A et al. Triglyceride-induced diabetes associated with familial lipoprotein lipase deficiency. Diabetes 1999; 48: 1258–1263.
Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G . Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR). J Clin Invest 1997; 100: 1166–1173.
Björntorp P, Berchtold P, Holm J, Larsson B . The glucose uptake of human adipose tissue in obesity. Eur J Clin Invest 1971; 1: 480–485.
Mårin P, Rebuffé-Scrive M, Smith U, Björntorp P . Glucose uptake in human adipose tissue. Metabolism 1987; 36: 1154–1160.
Virtanen KA, Lönnroth P, Parkkola R, Peltoniemi P, Asola M, Viljanen T et al. Glucose uptake and perfusion in subcutaneous and visceral adipose tissue during insulin stimulation in nonobese and obese humans. J Clin Endocrinol Metab 2002; 87: 3902–3910.
Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Manco M, Mingrone G . First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care 2009; 32: 375–380.
DeFronzo RA, Tobin JD, Andres R . Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237: E214–E223.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC . Homeostasis model assessment. Diabetologia 1985; 28: 412–419.
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G et al. Quantitative insulin sensitivity check index. A simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000; 85: 2402–2410.
Matsuda M, DeFronzo RA . Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22: 1462–1470.
Mari A, Pacini G, Murphy E, Ludvik B, Nolan J . A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care 2001; 24: 539–548.
Polonsky KS, Given BD, Hirsch L, Shapiro ET, Tillil H, Beebe C et al. Quantitative study of insulin secretion and clearance in normal and obese subjects. J Clin Invest 1988; 81: 435–441.
Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA, San Antonio metabolism study. Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia 2004; 47: 31–39.
Helge JW, Stallknecht B, Richter EA, Galbo H, Kiens B . Muscle metabolism during graded quadriceps exercise in man. J Physiol 2007; 581: 1247–1258.
Stevenson EJ, Williams C, Mash LE, Phillips B, Nute ML . Influence of high-carbohydrate mixed meals with different glycemic indexes on substrate utilization during subsequent exercise in women. Am J Clin Nutr 2006; 84: 354–360.
Huang X, Eriksson KF, Vaag A, Lehtovirta M, Hansson M, Laurila E et al. Insulin-regulated mitochondrial gene expression is associated with glucose flux in human skeletal muscle. Diabetes 1999; 48: 1508–1514.
Mitrou P, Boutati E, Lambadiari V, Maratou E, Papakonstantinou A, Komesidou V et al. Rates of glucose uptake in adipose tissue and muscle in vivo after a mixed meal in women with morbid obesity. J Clin Endocrinol Metab 2009; 94: 2958–2961.
Cnop M, Landchild MJ, Vidal J, Havel PJ, Knowles NG, Carr DR et al. The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations: distinct metabolic effects of two fat compartments. Diabetes 2002; 51: 1005–1015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Gniuli, D., Castagneto-Gissey, G., Iaconelli, A. et al. Fat mass largely contributes to insulin mediated glucose uptake in morbidly obese subjects. Int J Obes 34, 1726–1732 (2010). https://doi.org/10.1038/ijo.2010.99
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2010.99
Keywords
This article is cited by
-
Impact of high-fat feeding on basic helix–loop–helix transcription factors controlling enteroendocrine cell differentiation
International Journal of Obesity (2014)