Abstract
Objective:
Epicardial adipose tissue (EAT) is an interesting visceral fat pad with a particular location. EAT and subcutaneous adipose tissue (SAT) produce a wide range of adipokines. Some of them, including adiponectin and leptin, can influence the risk of development of diabetes and other associated metabolic and cardiovascular conditions. We sought to assess whether EAT and SAT adiponectin and leptin expression levels are different in diabetic patients with respect to nondiabetic subjects.
Subjects and methods:
We collected samples of EAT from 120 patients and samples of SAT from 88 of the same group of patients undergoing elective cardiac surgery for coronary artery bypass grafting (n=69) or other procedures (n=51). After RNA isolation, adiponectin and leptin expression levels were analyzed by real-time reverse transcriptase PCR. Plasma levels were determined in small subsamples of subjects. Baseline clinical and treatment data were obtained from medical records.
Results:
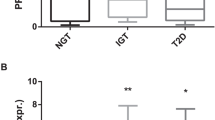
A total of 45 diabetic and 75 nondiabetic subjects were included in the study. Mean (s.d.) age was 70.1 (7.8) years and there were 32% women. EAT and SAT adiponectin and leptin mRNA expression levels were similar in the diabetic and the nondiabetic groups (EAT adiponectin 14.4 (4.3) vs 14.6 (3.4) arbitrary units (a.u.), P=0.79; SAT adiponectin 15.6 (4.7) vs 15.1 (3.9), P=0.54; EAT leptin 9.3 (interquartile range 2.5) vs 9.5 (1.9) a.u., P=0.72; SAT leptin 9.9 (3.6) vs 10.0 (2.5) a.u., P=0.96). These findings persisted after stratification for sex and coronary artery disease. Logistic regression models including possible confounders and a combination of diabetes and impaired fasting glucose as a dependent variable led to similar results. Plasma adiponectin levels were lower in diabetic patients, whereas leptin levels showed a nonsignificant trend.
Conclusion:
Diabetic and nondiabetic subjects express similar EAT and SAT adiponectin and leptin levels. Counter-regulatory mechanisms of adiponectin and leptin expression in patients with established diabetes might partly account for these findings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM . C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001; 286: 327–334.
Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003; 108: 2460–2466.
Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol 2006; 5: 1.
Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002; 288: 2709–2716.
Teijeira-Fernandez E, Eiras S, Grigorian-Shamagian L, Fernandez A, Adrio B, Gonzalez-Juanatey JR . Epicardial adipose tissue expression of adiponectin is lower in patients with hypertension. J Hum Hypertens 2008; 22: 856–863.
Eiras S, Teijeira-Fernandez E, Shamagian LG, Fernandez AL, Vazquez-Boquete A, Gonzalez-Juanatey JR . Extension of coronary artery disease is associated with increased IL-6 and decreased adiponectin gene expression in epicardial adipose tissue. Cytokine 2008; 43: 174–180.
Berg AH, Combs TP, Du X, Brownlee M, Scherer PE . The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 2001; 7: 947–953.
Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB . Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 2004; 291: 1730–1737.
Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace AM et al. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation 2006; 114: 623–629.
Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 2000; 20: 1595–1599.
Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet 2002; 360: 57–58.
Yamamoto Y, Hirose H, Saito I, Nishikai K, Saruta T . Adiponectin, an adipocyte-derived protein, predicts future insulin resistance: two-year follow-up study in Japanese population. J Clin Endocrinol Metab 2004; 89: 87–90.
Daimon M, Oizumi T, Saitoh T, Kameda W, Hirata A, Yamaguchi H et al. Decreased serum levels of adiponectin are a risk factor for the progression to type 2 diabetes in the Japanese Population: the Funagata study. Diabetes Care 2003; 26: 2015–2020.
Duncan BB, Schmidt MI, Pankow JS, Bang H, Couper D, Ballantyne CM et al. Adiponectin and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes 2004; 53: 2473–2478.
Mather KJ, Funahashi T, Matsuzawa Y, Edelstein S, Bray GA, Kahn SE et al. Adiponectin, change in adiponectin, and progression to diabetes in the Diabetes Prevention Program. Diabetes 2008; 57: 980–986.
Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW . Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004; 145: 2273–2282.
Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 2002; 110: 1093–1103.
Unger RH . Hyperleptinemia: protecting the heart from lipid overload. Hypertension 2005; 45: 1031–1034.
Cohen B, Novick D, Rubinstein M . Modulation of insulin activities by leptin. Science 1996; 274: 1185–1188.
Welsh P, Murray HM, Buckley BM, de Craen AJ, Ford I, Jukema JW et al. Leptin predicts diabetes but not cardiovascular disease: results from a large prospective study in an elderly population. Diabetes Care 2009; 32: 308–310.
Park HY, Kwon HM, Lim HJ, Hong BK, Lee JY, Park BE et al. Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp Mol Med 2001; 33: 95–102.
Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzman M, Brownlee M . Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem 2001; 276: 25096–25100.
Parhami F, Tintut Y, Ballard A, Fogelman AM, Demer LL . Leptin enhances the calcification of vascular cells: artery wall as a target of leptin. Circ Res 2001; 88: 954–960.
Santos-Alvarez J, Goberna R, Sanchez-Margalet V . Human leptin stimulates proliferation and activation of human circulating monocytes. Cell Immunol 1999; 194: 6–11.
Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, Gaw A et al. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS). Circulation 2001; 104: 3052–3056.
Sattar N, Wannamethee G, Sarwar N, Chernova J, Lawlor DA, Kelly A et al. Leptin and coronary heart disease: prospective study and systematic review. J Am Coll Cardiol 2009; 53: 167–175.
Brennan AM, Li TY, Kelesidis I, Gavrila A, Hu FB, Mantzoros CS . Circulating leptin levels are not associated with cardiovascular morbidity and mortality in women with diabetes: a prospective cohort study. Diabetologia 2007; 50: 1178–1185.
van Dielen FM, van’t Veer C, Schols AM, Soeters PB, Buurman WA, Greve JW . Increased leptin concentrations correlate with increased concentrations of inflammatory markers in morbidly obese individuals. Int J Obes Relat Metab Disord 2001; 25: 1759–1766.
Imagawa A, Funahashi T, Nakamura T, Moriwaki M, Tanaka S, Nishizawa H et al. Elevated serum concentration of adipose-derived factor, adiponectin, in patients with type 1 diabetes. Diabetes Care 2002; 25: 1665–1666.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2008; 31 (Suppl 1): S55–S60.
Iglesias MJ, Eiras S, Pineiro R, Lopez-Otero D, Gallego R, Fernandez AL et al. [Gender differences in adiponectin and leptin expression in epicardial and subcutaneous adipose tissue. Findings in patients undergoing cardiac surgery]. Rev Esp Cardiol 2006; 59: 1252–1260.
Liu YM, Lacorte JM, Viguerie N, Poitou C, Pelloux V, Guy-Grand B et al. Adiponectin gene expression in subcutaneous adipose tissue of obese women in response to short-term very low calorie diet and refeeding. J Clin Endocrinol Metab 2003; 88: 5881–5886.
Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, Clark LT et al. Adiponectin is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction in patients presenting with chest pain. Eur Heart J 2006; 27: 2300–2309.
Rathmann W, Herder C . Adiponectin and cardiovascular mortality: evidence for ‘reverse epidemiology’. Horm Metab Res 2007; 39: 1–2.
Iacobellis G, Barbaro G, Gerstein HC . Relationship of epicardial fat thickness and fasting glucose. Int J Cardiol 2008; 128: 424–426.
Singh N, Singh H, Khanijoun HK, Iacobellis G . Echocardiographic assessment of epicardial adipose tissue—a marker of visceral adiposity. Mcgill J Med 2007; 10: 26–30.
Iacobellis G, Pistilli D, Gucciardo M, Leonetti F, Miraldi F, Brancaccio G et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine 2005; 29: 251–255.
Saito S, Fujiwara T, Matsunaga T, Minagawa K, Fukui K, Fukuda I et al. Increased adiponectin synthesis in the visceral adipose tissue in men with coronary artery disease treated with pravastatin: a role of the attenuation of oxidative stress. Atherosclerosis 2008; 199: 378–383.
Chu CH, Lee JK, Lam HC, Lu CC, Sun CC, Wang MC et al. Atorvastatin does not affect insulin sensitivity and the adiponectin or leptin levels in hyperlipidemic type 2 diabetes. J Endocrinol Invest 2008; 31: 42–47.
Koh KK, Quon MJ, Han SH, Ahn JY, Jin DK, Kim HS et al. Vascular and metabolic effects of combined therapy with ramipril and simvastatin in patients with type 2 diabetes. Hypertension 2005; 45: 1088–1093.
Forst T, Pfutzner A, Lubben G, Weber M, Marx N, Karagiannis E et al. Effect of simvastatin and/or pioglitazone on insulin resistance, insulin secretion, adiponectin, and proinsulin levels in nondiabetic patients at cardiovascular risk—the PIOSTAT Study. Metabolism 2007; 56: 491–496.
Takagi T, Matsuda M, Abe M, Kobayashi H, Fukuhara A, Komuro R et al. Effect of pravastatin on the development of diabetes and adiponectin production. Atherosclerosis 2008; 196: 114–121.
Norhammar A, Tenerz A, Nilsson G, Hamsten A, Efendic S, Ryden L et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet 2002; 359: 2140–2144.
Bartnik M, Ryden L, Ferrari R, Malmberg K, Pyorala K, Simoons M et al. The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe. The Euro Heart Survey on diabetes and the heart. Eur Heart J 2004; 25: 1880–1890.
Acknowledgements
This study was supported by Hospital Clínico Universitario de Santiago de Compostela (Santiago de Compostela, Spain) and by a grant from Xunta de Galicia (PGIDIT07PXIB918092PR). Dr S Eiras is a researcher within the Isidro Parga Pondal Program (Xunta de Galicia, Santiago de Compostela, Spain).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Teijeira-Fernandez, E., Eiras, S., Grigorian-Shamagian, L. et al. Diabetic and nondiabetic patients express similar adipose tissue adiponectin and leptin levels. Int J Obes 34, 1200–1208 (2010). https://doi.org/10.1038/ijo.2010.30
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2010.30