Abstract
Objective:
Several lines of evidence indicate that nutrient restriction during perinatal development sensitizes the offspring to the development of obesity, insulin resistance and cardiovascular disease in adulthood via the programming of hyperphagia and reduced energy expenditure. Given the link between the circadian clock and energy metabolism, and the resetting action of food on the circadian clock, in this study, we have investigated whether perinatal undernutrition affects the circadian expression rhythms of genes regulating food intake in the hypothalamus and energy metabolism in the liver.
Design:
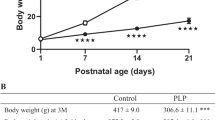
Pregnant Sprague-Dawley rats were fed ad libitum either a control (20% protein) or a low-protein (8% protein) diet throughout pregnancy and lactation. At weaning, pups received a standard diet and at 17 and 35 days of age, their daily patterns of gene expression were analyzed by real-time quantitative PCR experiments.
Results:
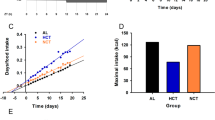
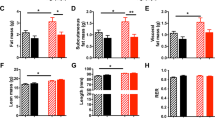
17-day-old pups exposed to perinatal undernutrition exhibited significant alterations in the circadian expression profile of the transcripts encoding diverse genes regulating food intake, the metabolic enzymes fatty acid synthase and glucokinase as well as the clock genes BMAL1 and Period1. These effects persisted after weaning, were associated with hyperphagia and mirrored the results of the behavioral analysis of feeding. Thus, perinatally undernourished rats exhibited an increased hypothalamic expression of the orexigenic peptides agouti-related protein and neuropeptide Y. Conversely, the mRNA levels of the anorexigenic peptides pro-opiomelanocortin and cocaine and amphetamine-related transcripts were decreased.
Conclusion:
These observations indicate that the circadian clock undergoes nutritional programming. The programming of the circadian clock may contribute to the alterations in feeding and energy metabolism associated with malnutrition in early life, which might promote the development of metabolic disorders in adulthood.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hastings MH, Reddy AB, Maywood ES . A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci 2003; 4: 649–661.
Schibler U, Sassone-Corsi P . A web of circadian pacemakers. Cell 2002; 111: 919–922.
Welsh DK, Logothetis DE, Meister M, Reppert SM . Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 1995; 14: 697–706.
Feillet CA, Mendoza J, Albrecht U, Pévet P, Challet E . Forebrain oscillators ticking with different clock hands. Mol Cell Neurosci 2008; 37: 209–221.
Guilding C, Hughes A, Brown T, Namvar S, Piggins H . An ensemble of novel circadian oscillators in the mouse mediobasal hypothalamus. Comp Biochem Physiol A Comp Physiol 2008; 150: S149.
Wisor JP, Pasumarthi RK, Gerashchenko D, Thompson CL, Pathak S, Sancar A et al. Sleep deprivation effects on circadian clock gene expression in the cerebral cortex parallel electroencephalographic differences among mouse strains. J Neurosci 2008; 28: 7193–7201.
Wakamatsu H, Yoshinobu Y, Aida R, Moriya T, Akiyama M, Shibata S . Restricted-feeding-induced anticipatory activity rhythm is associated with a phase-shift of the expression of mPer1 and mPer2 mRNA in the cerebral cortex and hippocampus but not in the suprachiasmatic nucleus of mice. Eur J Neurosci 2001; 13: 1190–1196.
Angeles-Castellanos M, Mendoza J, Escobar C . Restricted feeding schedules phase shift daily rhythms of c-Fos and protein Per1 immunoreactivity in corticolimbic regions in rats. Neuroscience 2007; 144: 344–355.
Peirson SN, Butler JN, Duffield GE, Takher S, Sharma P, Foster RG . Comparison of clock gene expression in SCN, retina, heart, and liver of mice. Biochem Biophys Res Commun 2006; 351: 800–807.
Yanagihara H, Ando H, Hayashi Y, Obi Y, Fujimura A . High-fat feeding exerts minimal effects on rhythmic mRNA expression of clock genes in mouse peripheral tissues. Chronobiol Int 2006; 23: 905–914.
Pardini L, Kaeffer B, Trubuil A, Bourreille A, Galmiche JP . Human intestinal circadian clock: expression of clock genes in colonocytes lining the Crypt. Chronobiol Int 2005; 22: 951–961.
Sládek M, Rybová M, Jindráková Z, Zemanová Z, Polidarová L, Mrnka L et al. Insight into the circadian clock within rat colonic epithelial cells. Gastroenterology 2007; 133: 1240–1249.
Hoogerwerf WA, Hellmich HL, Cornélissen G, Halberg F, Shahinian VB, Bostwick J et al. Clock gene expression in the murine gastrointestinal tract: endogenous rhythmicity and effects of a feeding regimen. Gastroenterology 2007; 133: 1250–1260.
Oishi K, Miyazaki K, Kadota K, Kikuno R, Nagase T, Atsumi G-I et al. Genome-wide expression analysis of mouse liver reveals CLOCK-regulated circadian output genes. J Biol Chem 2003; 278: 41519–41527.
Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 2002; 109: 307–320.
Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M et al. Nuclear receptor expression links the circadian clock to metabolism. Cell 2006; 126: 801–810.
Fukagawa K, Sakata T, Yoshimatsu H, Fujimoto K, Uchimura K, Asano C . Advance shift of feeding circadian rhythm induced by obesity progression in Zucker rats. Am J Physiol Regul Integr Comp Physiol 1992; 263: R1169–R1175.
Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science 2005; 308: 1043–1045.
Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol 2004; 2: e377.
Suwazono Y, Dochi M, Sakata K, Okubo Y, Oishi M, Tanaka K et al. A longitudinal study on the effect of shift work on weight gain in male japanese workers. Obesity 2008; 16: 1887–1893.
Garaulet M, Madrid JA . Chronobiology, genetics and metabolic syndrome. Curr Opin Lipidol 2009; 20: 127–134.
Abe H, Kida M, Tsuji K, Mano T . Feeding cycles entrain circadian rhythms of locomotor activity in CS mice but not in C57BL/6J mice. Physiol Behav 1989; 45: 397–401.
Boulos Z, Rosenwasser AM, Terman M . Feeding schedules and the circadian organization of behavior in the rat. Behav Brain Res 1980; 1: 39–65.
Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U . Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 2000; 14: 2950–2961.
Stokkan K-A, Yamazaki S, Tei H, Sakaki Y, Menaker M . Entrainment of the circadian clock in the liver by feeding. Science 2001; 291: 490–493.
Castillo MR, Hochstetler KJ, Tavernier RJ, Greene DM, Bult-Ito A . Entrainment of the master circadian clock by scheduled feeding. Am J Physiol Regul Integr Comp Physiol 2004; 287: R551–R555.
Ravelli GP, Stein ZA, Susser MW . Obesity in young men after famine exposure in utero and early infancy. N Engl J Med 1976; 295: 349–353.
Barker DJ, Bull AR, Osmond C, Simmonds SJ . Fetal and placental size and risk of hypertension in adult life. BMJ 1990; 301: 259–262.
Yajnik CS . Early life origins of insulin resistance and Type 2 diabetes in India and other Asian Countries. J Nutr 2004; 134: 205–210.
Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD . Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab 2000; 279: E83–E87.
Bellinger L, Sculley DV, Langley-Evans SC . Exposure to undernutrition in fetal life determines fat distribution, locomotor activity and food intake in ageing rats. Int J Obes 2006; 30: 729–738.
Lopes de Souza S, Orozco-Solís R, Grit I, Manhães de Castro R, Bolaños-Jiménez F . Perinatal protein restriction reduces the inhibitory action of serotonin on food intake. Eur J Neurosci 2008; 27: 1400–1408.
Desai M, Gayle D, Babu J, Ross MG . Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol 2005; 288: R91–R96.
Zambrano E, Bautista CJ, Deás M, Martínez-Samayoa PM, González-Zamorano M, Ledesma H et al. A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J Physiol 2006; 571: 221–230.
Bieswal F, Ahn M-T, Reusens B, Holvoet P, Raes M, Rees WD et al. The importance of catch-up growth after early malnutrition for the programming of obesity in male Rats. Obesity 2006; 14: 1330–1343.
Krechowec SO, Vickers M, Gertler A, Breier BH . Prenatal influences on leptin sensitivity and susceptibility to diet-induced obesity. J Endocrinol 2006; 189: 355–363.
Desai M, Babu J, Ross MG . Programmed metabolic syndrome: prenatal undernutrition and postweaning overnutrition. Am J Physiol Regul Integr Comp Physiol 2007; 293: R2306–R2314.
Erhuma A, Salter AM, Sculley DV, Langley-Evans SC, Bennett AJ . Prenatal exposure to a low-protein diet programs disordered regulation of lipid metabolism in the aging rat. Am J Physiol Endocrinol Metab 2007; 292: E1702–E1714.
Gopalakrishnan GS, Gardner DS, Dandrea J, Langley-Evans SC, Pearce S, Kurlak LO et al. Influence of maternal pre-pregnancy body composition and diet during early-mid pregnancy on cardiovascular function and nephron number in juvenile sheep. Br J Nutr 2005; 94: 938–947.
McMullen S, Langley-Evans SC . Maternal low-protein diet in rat pregnancy programs blood pressure through sex-specific mechanisms. Am J Physiol Regul Integr Comp Physiol 2005; 288: R85–R90.
Gluckman PD, Hanson MA, Pinal C . The developmental origins of adult disease. Matern Child Nutr 2005; 1: 130–141.
Barker DJ . The fetal and infant origins of adult disease. BMJ 1990; 301: 1111.
Gluckman PD, Hanson MA . Developmental and epigenetic pathways to obesity: an evolutionary-developmental perspective. Int J Obes 2008; 32: S62–S71.
Hales CN, Barker DJP . The thrifty phenotype hypothesis: type 2 diabetes. Br Med Bull 2001; 60: 5–20.
Gluckman PD, Hanson MA . Living with the past: evolution, development, and patterns of disease. Science 2004; 305: 1733–1736.
Orozco-Sólis R, Lopes de Souza S, Barbosa Matos RJ, Grit I, Le Bloch J, Nguyen P et al. Perinatal undernutrition-induced obesity is independent of the developmental programming of feeding. Physiol Behav 2009; 96: 481–492.
Sladek M, Jindrakova Z, Bendova Z, Sumova A . Postnatal ontogenesis of the circadian clock within the rat liver. Am J Physiol Regul Integr Comp Physiol 2007; 292: R1224–R1229.
Sumova A, Bendova Z, Sladek M, Kovacikova Z, El-Hennamy R, Laurinova K et al. The Rat circadian clockwork and its photoperiodic entrainment during development. Chronobiol Int 2006; 23: 237–243.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2-[Delta][Delta]CT Method. Methods 2001; 25: 402–408.
Cherala G, Shapiro BH, D’Mello AP . Two low protein diets differentially affect food consumption and reproductive performance in pregnant and lactating rats and long-term growth in their offspring. J Nutr 2006; 136: 2827–2833.
Wilson JF . Effects of pregnancy, sucrose, and various low-protein diets on the eating behavior of rats. Physiol Behav 1997; 62: 779–782.
Kanarek RB, Schoenfeld PM, Morgane PJ . Maternal malnutrition in the rat: effects on food intake and body weight. Physiol Behav 1986; 38: 509–515.
Froy O . The relationship between nutrition and circadian rhythms in mammals. Front Neuroendocrinol 2007; 28: 61–71.
Landry GJ, Mistlberger RE . Food entrainment: methodological issues. J Biol Rhythms 2007; 22: 484–487.
Orozco-Sólis R, Barbosa Matos RJ, Guzmán-Quevedo O, Lopes de Souza S, Bihouée A., Houlgatte R. et al. Nutritional programming in the rat is linked to long-lasting changes in nutrient sensing and energy homeostasis in the hypothalamus. PLoS ONE 2010; 5: e13537.
Akashi M, Takumi T . The orphan nuclear receptor ROR[alpha] regulates circadian transcription of the mammalian core-clock Bmal1. Nat Struct Mol Biol 2005; 12: 441–448.
Liu C, Li S, Liu T, Borjigin J, Lin JD . Transcriptional coactivator PGC-1[agr] integrates the mammalian clock and energy metabolism. Nature 2007; 447: 477–481.
McNamara P, Seo S-B, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA . Regulation of CLOCK and MOP4 by Nuclear Hormone Receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell 2001; 105: 877–889.
Challet E, Pevet P, Vivien-Roels B, Malan A . Phase-advanced daily rhythms of melatonin, body temperature, and locomotor activity in food-restricted rats fed during daytime. J Biol Rhythms 1997; 12: 65–79.
Mendoza J, Prevet P, Challet E . Circadian and photic regulation of clock and clock-controlled proteins in the suprachiasmatic nuclei of calorie-restricted mice. Eur J Neurosci 2007; 25: 3691–3701.
Mendoza J, Graff C, Dardente H, Pevet P, Challet E . Feeding cues alter clock gene oscillations and photic responses in the suprachiasmatic nuclei of mice exposed to a light/dark cycle. J Neurosci 2005; 25: 1514–1522.
Weaver DR . The suprachiasmatic nucleus: a 25-year retrospective. J Biol Rhythms 1998; 13: 100–112.
Mirmiran M, Ariagno RL . Influence of light in the NICU on the development of circadian rhythms in preterm infants. Semin Perinatol 2000; 24: 247–257.
Rivkees SA . Developing circadian rhythmicity in infants. Pediatrics 2003; 112: 373–381.
Mirmiran M, Maas YGH, Ariagno RL . Development of fetal and neonatal sleep and circadian rhythms. Sleep Med Rev 2003; 7: 321–334.
Recio J, Miguez JM, Buxton OM, Challet E . Synchronizing circadian rhythms in early infancy. Med Hypotheses 1997; 49: 229–234.
Rivkees SA . Emergence and influences of circadian rhythmicity in infants. Clin Perinatol 2004; 31: 217–228.
Acknowledgements
This work was supported by INRA through a Young Team Action and by l’Agence National de la Recherche (ANR-05-PNRA-009–02). R Orozco-Sólis is a doctoral fellow from the Mexican Consejo Nacional de Ciencia y Tecnología (CONACYT). S Lopes de Souza and Rhowena Matos are the recipient of a post-doctoral fellowship from La Region Pays de la Loire. The authors gratefully acknowledge M Jean Louis Lescure for his assistance with the care of the animals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Orozco-Solís, R., Matos, R., Lopes de Souza, S. et al. Perinatal nutrient restriction induces long-lasting alterations in the circadian expression pattern of genes regulating food intake and energy metabolism. Int J Obes 35, 990–1000 (2011). https://doi.org/10.1038/ijo.2010.223
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2010.223
Keywords
This article is cited by
-
Circadian behavior of adult mice exposed to stress and fluoxetine during development
Psychopharmacology (2017)
-
Taurine Treatment Modulates Circadian Rhythms in Mice Fed A High Fat Diet
Scientific Reports (2016)
-
Taurine supplementation preserves hypothalamic leptin action in normal and protein-restricted mice fed on a high-fat diet
Amino Acids (2015)
-
Developmental Origins of Obesity: Programmed Adipogenesis
Current Diabetes Reports (2013)