Abstract
Introduction:
We previously demonstrated in an animal model that steatosis, in the absence of fibrosis, induces a significant rise in portal pressure, indicating substantial changes in liver hemodynamics. As assessment of portal pressure is an invasive procedure, non-invasive parameters are needed to identify patients at risk.
Aims:
To study the portal pressure in nonalcoholic fatty liver disease patients and to identify factors that are possibly related to steatosis-induced changes in liver hemodynamics.
Materials and methods:
Patients presenting with a problem of overweight or obesity, and in whom non-invasive investigations showed signs of liver involvement, were proposed for transjugular hepatic vein catheterization and liver biopsy. The biopsy was scored according to the Nonalcoholic Steatohepatitis Clinical Research Network Scoring System.
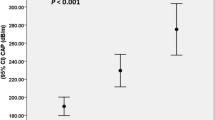
Results:
A total of 50 consecutive patients were studied. Their mean age was 47.9±1.8 years; 31 (62%) were female. Hepatic venous pressure gradient was normal in 36 (72%) and elevated in 14 (28%) patients. The degree of steatosis was the only histological parameter that differed significantly between the two groups (P=0.016), and was a predictor of the presence of portal hypertension (PHT) in regression analysis (P=0.010). Comparing normal versus portal hypertensive patients, waist circumference (117±2 versus 128±4 cm, P=0.005), waist–hip ratio (0.96±0.06 versus 1.04±0.03, P=0.003), visceral fat (229±15 versus 292±35 cm2, P=0.022), fasting insulin (15.4±1.7 versus 21.8±2.4 μU ml−1, P=0.032), fasting c-peptide (1.22±0.06 versus 1.49±0.09 nmol l−1, P=0.035) and homeostasis model assessment–insulin resistance (HOMA IR) (3.28±0.29 versus 4.81±0.57, P=0.019) were significantly higher. Age, gender, liver enzymes, ferritin and high-sensitive C-reactive protein were not significantly different. In regression analysis, waist circumference (P=0.008) and HOMA IR (P=0.043) were independent predictors of PHT.
Conclusions:
Estimates of both visceral adiposity and IR are predictors for the presence of PHT, related to the degree of steatosis, and may help in identifying patients who are at risk of developing steatosis-related complications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Angulo P . Non-alcoholic fatty liver disease. N Engl J Med 2002; 346: 1221–1231.
Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P et al. Expanding the natural history of non-alcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002; 123: 134–143.
Selzner M, Clavien P . Fatty liver in liver transplantation and surgery. Semin Liver Dis 2001; 21: 105–113.
Behrns K, Tsiotos G, DeSouza N, Krishna M, Ludwig J, Nagorney D . Hepatic steatosis as a potential risk factor for major hepatic resections. J Gastrointest Surg 1998; 2: 292–298.
Rao M, Papreddy K, Abecassis M, Hashimoto T . Regeneration of liver with marked fatty change following partial hepatectomy in rats. Dig Dis Sci 2001; 46: 1821–1826.
Selzner M, Rüdiger H, Sindram D, Madden J, Clavien . Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology 2000; 32: 1281–1288.
Choi S, Diehl AM . Role of inflammation in non-alcoholic steatohepatitis. Curr Opin Gastroenterol 2005; 21: 702–707.
Seifalian A, Chidambaram V, Rolles K, Davidson B . In vivo demonstration of impaired microcirculation in steatotic human liver grafts. Liver Transpl Surg 1998; 4: 71–77.
McCuskey RS, Ito Y, Robertson GR, McCuskey MK, Perry M, Farrell GC . Hepatic microvascular dysfunction during evolution of dietary steatohepatitis in mice. Hepatology 2004; 40: 386–393.
Francque S, Wamutu S, Chatterjee S, Van Marck E, Herman A, Ramon A et al. Non-alcoholic steatohepatitis induces non-fibrosis related portal hypertension associated with splanchnic vasodilation and signs of a hyperdynamic circulation in vitro and in vivo in a rat model. Liver Int 2010; 30: 365–375.
Lebrec D, Moreau R . Pathogenesis of portal hypertension. Eur J Gastroenterol Hepatol 2001; 13: 309–311.
Laleman W, Van Landeghem L, Wilmer A, Fevery J, Nevens F . Portal hypertension: from pathophysiology to clinical practice. Liver Int 2005; 25: 1070–1090.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC . Homeostasis model assessment: insulin resistance and β-cell functioning from fasting glucose and insulin concentrations in men. Diabetology 1985; 28: 412–419.
van der Kooy K, Seidell JC . Techniques for the measurement of visceral fat: a practical guide. Int J Obes Relat Metab Disord 1993; 17: 187–196.
Bucombe JR, Hilson AJW . Radionuclide investigations of the liver. Oxford Textbook of Clinical Hepatology, 2nd edn. Oxford University Press: Oxford, UK, 1999. pp 579–588.
Merkel C, Bolognesi M, Bellon S, Bianca S, Honisch B, Lampe H et al. Aminopyrine breath test in the prognostic evaluation of patients with cirrhosis. Gut 1992; 33: 836–842.
Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003; 37: 917–923.
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285: 2486–2497.
Alberti KG, Zimmet P, Shaw J, IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—a new worldwide definition. Lancet 2005; 366: 1059–1062.
Farrell G, George J, de la M Hall P, McCullough AJ . An introduction to NASH and related fatty liver disorders. Fatty Liver Disease; NASH and related disorders. Blackwell Publishing: USA, 2005; 1–12.
Cosgrove DO . Liver Anatomy. In: Cosgrove D, Meire H, Dewbury K (eds). Abdominal and General Ultrasound vol. 1, 3rd edn. Churchill Livingstone: New York, 1994. pp 227–242.
Bellentani S, Saccoccio G, Masutti F, Croce LS, Brandi G, Sasso F et al. Prevalence of and risk factors for hepatic steatosis in northern Italy. Ann Intern Med 2000; 132: 112–117.
Dardenne AN . The spleen. In: Cosgrove D, Meire H, Dewbury K (eds). Abdominal and General Ultrasound vol. 1, 3rd edn. Churchill Livingstone: New York, 1994. pp 351–366.
Pratt DS, Kaplan MM . Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med 2000; 342: 1266–1271.
Prati D, Taioli E, Zanella A, Della Torre E, Buttelli S, Del Vecchio E et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002; 137: 1–10.
Joy D, Thava VR, Scott BB . Diagnosis of fatty liver disease: is biopsy necessary? Eur J Gastroenterol Hepatol 2003; 15: 539–543.
Kichian K, McLean R, Gramlich LM, Bailey RJ, Bain VG . Nonalcoholic fatty liver disease in patients investigated for elevated liver enzymes. Can J Gastroenterol 2003; 17: 38–42.
Tawalkar JA . Motion—all patients with NASH need to have a liver biopsy: arguments for the motion. Can J Gastroenterol 2002; 16: 718–721.
Campbell MS, Reddy KR . The evolving role of liver biopsy. Aliment Pharmacol Ther 2004; 20: 249–259.
Kalambokis G, Manousou P, Vibhakorn S, Marelli L, Cholongitas E, Senzolo M et al. Transjugular liver biopsy—indications, adequacy, quality of specimens, and complications—A systematic review. J Hepatology 2007; 47: 284–294.
Lebrec D, Godfarb G, Degott C, Rueff B, Benhamou JP . Transvenous liver biopsy: an experience based on 1000 hepatic tissue samplings with this procedure. Gastroenterology 1982; 83: 338–340.
Groszmann R, Vorobioff JD, Gao H . Measurement of portal pressure: when, how and why to do it. Clin Liver Dis 2006; 10: 499–512.
Bosch J, Garcia-Pagan JC, Berzigotti A, Abraldes JG . Measurement of portal pressure and its role in the management of chronic liver disease. Semin Liv Dis 2006; 26: 348–362.
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW et al. for the nonalcoholic steatohepatitis clinical research network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41: 1313–1321.
World Health Organisation (WHO). Obesity: preventing and managing the global epidemic report of a WHO consultation. WHO Technical Report Series 894. World Health Organisation: Geneva, 2000.
Alberti KG, Zimmer P . Definition, diagnosis and classification of diabetes mellitus and its complications. Part I. Diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med 1998; 15: 539–553.
Ducimetiere P, Eschwege E, Papoz L, Richard JL, Claude JR, Rosselin G . Relationship of plasma insulin levels to the incidence of myocardial infarction and coronary heart disease mortality in a middle-aged population. Diabetologia 1980; 19: 205–210.
Puoti C, Bellis L . Steatosis and portal hypertension. Eur Rev Med Pharmacol Sci 2005; 9: 285–290.
Després JP, Lemieux I, Prud’homme D . Treatment of obesity: need to focus on high risk abdominally obese patients. Br Med J 2001; 322: 716–720.
Clark JM, Brancati FL, Diehl AM . Nonalcoholic fatty liver disease. Gastroenterology 2002; 122: 1649–1657.
Rinella M, Alonso E, Rao S, Whitington P, Fryer J, Abecassis M et al. Body mass index as a predictor of hepatic steatosis in living liver donors. Liver Transplant 2001; 7: 409–413.
Egushi Y, Egushi T, Mizuka T, Ide Y, Yasutake T, Iwakini R et al. Visceral fat accumulation and insulin resistance are important factors in non-alcoholic liver disease. J Gastroenterol 2006; 41: 462–469.
Stranges S, Dorn JM, Muti P, Freudenheim JL, Farinaro E, Russell M et al. Body fat distribution, relative weight, and liver enzyme levels: a population-based study. Hepatology 2004; 39: 754–763.
Ahima RS, Flier JS . Adipose tissue as an endocrine organ. Trends Endocrinol Metab 2000; 11: 327–332.
Jarrar MH, Baranova A, Collantes R, Ranard B, Stepanova M, Bennett C et al. Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2008; 27: 412–421.
Neuschwander-Tetri BA . Fatty liver and the metabolic syndrome. Curr Opin Gastroenterol 2007; 23: 193–198.
Bugianesi E, McCullough AJ, Marchesini G . Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology 2005; 42: 987–1000.
Verrijken A, Francque S, Mertens I, Talloen M, Peiffer F, Van Gaal L . Visceral adipose tissue and inflammation correlate with elevated liver tests in a cohort of overweight and obese patients. Int J Obes 2010; 34: 899–907.
Francque S . Non-alcoholic fatty liver disease (NAFLD) and Non-alcoholic Steatohepatitis (NASH). In: Van Damme P, van Herck K, Michielsen P, Francque S, Shouval D (eds). Chronic Hepatitis & Other Liver Disease. Oxford Textbook of Public Health 5th edn. Oxford University Press: Oxford, UK, 2009. (Chapter 9) 16, pp 1249–1263.
de Marco R, Locatelli F, Zoppini G, Verlato G, Bonora E, Muggeo M . Cause-specific mortality in type 2 diabetes. The Verona Diabetes Study. Diabetes Care 1999; 22: 756–761.
Abrams GA, Kunde SS, Lazenby AJ, Clements RH . Portal fibrosis and hepatic steatosis in morbidly obese subjects: a spectrum of non-alcoholic fatty liver disease. Hepatology 2004; 40: 475–483.
Reaven GM . Role of insulin resistance in human diabetes. Diabetes 1988; 37: 1595–1607.
Acknowledgements
This work is part of the project ‘Hepatic and adipose tissue and functions in the metabolic syndrome’ (HEPADIP), which is supported by the European Commission as an Integrated Project under the 6th Framework Program (Contract LSHM-CT-2005-018734).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Francque, S., Verrijken, A., Mertens, I. et al. Visceral adiposity and insulin resistance are independent predictors of the presence of non-cirrhotic NAFLD-related portal hypertension. Int J Obes 35, 270–278 (2011). https://doi.org/10.1038/ijo.2010.134
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2010.134