Abstract
Objective:
To determine the efficacy of a long-acting oxyntomodulin (OXM) analogue, OXM6421, in inhibiting food intake and decreasing body weight in lean and diet-induced obese (DIO) rodents.
Research design and methods:
The glucagon-like peptide-1 (GLP-1) receptor binding affinity and efficacy, sensitivity to enzymatic degradation in vitro and persistence in the circulation after peripheral administration were investigated for OXM6421 and compared with native OXM. The chronic effect of OXM6421 on food intake, body weight and energy expenditure was examined in lean rats, and its anti-obesity potential was evaluated in DIO mice.
Results:
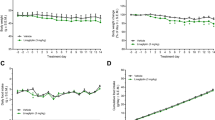
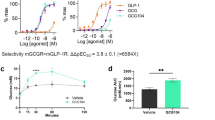
OXM6421 showed enhanced GLP-1 receptor binding affinity and cyclic adenosine monophosphate (cAMP) stimulation, and higher resistance to enzymatic degradation by dipeptidyl peptidase IV (DPP-IV) and neutral endopeptidase (NEP) compared with native OXM. OXM6421 persisted longer in the circulation than OXM after peripheral administration. Acute administration of OXM6421 potently inhibited food intake in lean rodents, with cumulative effects lasting up to 24 h. In lean rats, daily subcutaneous (s.c.) administration of OXM6421 caused greater weight loss than the pair-fed animals, and a higher rate of oxygen consumption than both the pair-fed and the saline controls. In DIO mice, continuous s.c. infusion of OXM6421 resulted in a significant weight loss, accompanied by an improvement in glucose homeostasis and an increase in circulating adiponectin levels. Once-daily s.c. administration of OXM6421 for 21 days caused sustained weight loss in DIO mice.
Conclusion:
OXM6421 induces negative energy balance in both lean and obese rodents, suggesting that long-acting OXM analogues may represent a potential therapy for obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50–71 years old. N Engl J Med 2006; 355: 763–778.
Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM . Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 2004; 291: 2847–2850.
Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB . Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med 2002; 162: 1867–1872.
Solomam CG, Manson JE . Obesity and mortality: a review of the epidemiologic data. Am J Clin Nutr 1997; 66: 1044S–1050S.
Rucker D, Padwal R, Li SK, Curioni C, Lau DCW . Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ 2007; 335: 1194–1199.
Näslund E, Grybäck P, Hellström PM, Jacobsson H, Holst JJ, Theodorsson E et al. Gastrointestinal hormones and gastric emptying 20 years after jejunoileal bypass for massive obesity. Int J Obes 1997; 21: 387–392.
Colquitt JL, Picot J, Loveman E, Clegg AJ . Surgery for obesity. Cochrane Database Syst Rev 2009; 2: CD003641.
Murphy KG, Bloom SR . Gut hormones and the regulation of energy homeostasis. Nature 2006; 444: 854–859.
Ghatei MA, Uttenthal LO, Christofides ND, Bryant MG, Bloom SR . Molecular forms of human enteroglucagon in tissue and plasma: plasma responses to nutrient stimuli in health and in disorders of the upper gastrointestinal tract. J Clin Endocrinol Metab 1983; 57: 488–495.
Dakin CL, Small CJ, Batterham RL, Neary NM, Cohen MA, Patterson M et al. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology 2004; 145: 2687–2695.
Dakin CL, Small CJ, Park AJ, Seth A, Ghatei MA, Bloom SR . Repeated ICV administration of oxyntomodulin causes a greater reduction in body weight gain than in pair-fed rats. Am J Physiol Endocrilol Metab 2002; 283: E1173–E1177.
Wynne K, Park AJ, Small CJ, Patterson M, Ellis SM, Murphy KG et al. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects. Diabetes 2005; 54: 2390–2395.
Schjoldager BT, Baldissera FG, Mortensen PE, Holst JJ, Christiansen J . Oxyntomodulin: a potential hormone from the distal gut. Pharmacokinetics and effects on gastric acid and insulin secretion in man. Eur J Clin Invest 1988; 18: 499–503.
Kervran A, Dubrasquet M, Blache P, Martinez J, Bataille D . Metabolic clearance rates of oxyntomodulin and glucagon in the rat: contribution of the kidney. Regul Pept 1990; 31: 41–52.
Zhu L, Tamvakopoulos C, Xie D, Dragovic J, Shen X, Fenyk-Melody JE et al. The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides. J Bio Chem 2003; 278: 22418–22423.
Hupe-Sodmann K, McGregor GP, Bridenbaugh R, Goke R, Goke B, Thole H et al. Characterisation of the processing by human neutral endopeptidase 24.11 of GLP-1 (7–36) amide and comparison of the substrate specificity of the enzyme for other glucagon-like peptides. Regul Pept 1995; 58: 149–156.
Plamboeck A, Holst JJ, Carr RD, Deacon CF . Neutral endopeptidase 24.11 and dipeptidyl peptidase IV are both mediators of the degradation of glucagon-like peptide 1 in the anaesthetised pig. Diabetologia 2005; 48: 1882–1890.
Druce M, Minnion J, Field B, Patel S, Cuenco J, Tilby M et al. Investigation of structure-activity relationships of Oxyntomodulin (Oxm) using Oxm analogues. Endocrinology 2009; 150: 1712–1722.
Johnston SL, Souter DM, Tolkamp BJ, Gordon IJ, Illius AW, Kyriazakis I et al. Intake compensates for resting metabolic rate variation in female C57BL/6J mice fed high-fat diets. Obesity (Silver Spring) 2007; 15: 600–606.
Qotthardt M, Lalyko G, van Eerd-Vismale J, Keil B, Schurrat T, Hower M et al. A new technique for in vivo imaging of specific GLP-1 binding sites: first results in small rodents. Regul Pept 2006; 137: 162–167.
Bhogal R, Smith DM, Bloom SR . Solubilization of binding sites for IAPP and CGRP from rat lung. Peptides 1994; 15: 1383–1390.
Stanley SA, Small CJ, Kim MS, Heath MM, Seal LJ, Russell SH et al. Agouti related peptide (Agrp) stimulates the hypothalamo pituitary gonadal axis in vivo and in vitro in male rats. Endocrinology 1999; 140: 5459–5462.
Murphy KG, Abbott CR, Mahmoudi M, Hunter R, Gardiner JV, Rossi M et al. Quantification and synthesis of cocaine- and amphetamine-regulated transcript peptide (79–102)-like immunoreactivity and mRNA in rat tissues. J Endocrinol 2000; 166: 659–668.
Seal LJ, Small CJ, Dhillo WS, Stanley SA, Abbott CR, Ghatei MA et al. PRL-releasing peptide inhibits food intake in male rats via the dorsomedial hypothalamic nucleus and not the paraventricular hypothalamic nucleus. Endocrinology 2001; 142: 4236–4243.
Allen JM, Yeats JC, Adrian TE, Bloom SR . Radioimmunoassay of neuropeptide Y. Regul Pept 1984; 8: 61–70.
Baggio LL, Huang Q, Brown TJ, Drucker DJ . Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology 2004; 127: 546–558.
Wynne K, Park AJ, Small CJ, Meeran K, Ghatei MA, Frost GS et al. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. Int J Obes (Lond) 2006; 30: 1729–1736.
Schwartz MW, Woods SC, Porte Jr D, Seeley RJ, Baskin DG . Central nervous system control of food intake. Nature 2000; 404: 661–671.
Yasuda T, Masaki T, Kakuma T, Yoshimatsu H . Hypothalamic melanocortin system regulates sympathetic nerve activity in brown adipose tissue. Exp Biol Med (Maywood) 2004; 229: 235–239.
Hoggard N, Rayner DV, Johnston SL, Speakman JR . Peripherally administered [Nle4,D-Phe7]-alpha-melanocyte stimulating hormone increases resting metabolic rate, while peripheral agouti-related protein has no effect, in wild type C57BL/6 and ob/ob mice. J Mol Endocrinol 2004; 33: 693–703.
Pocai A, Carrington PE, Adams JR, Michael W, Eiermann G, Zhu L et al. GLP-1/GCGR dual agonism reverses obesity in mice. Diabetes 2009; 58: 2258–2266.
Day JW, Ottaway N, Patterson JT, Gelfanov V, Smiley D, Gidda J et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol 2009; 5: 749–757.
Moraes RC, Blondet A, Birkenkamp-Demtroeder K . Study of the alteration of gene expression in adipose tissue of diet-induced obese mice by microarray and reverse transcription-polymerase chain reaction analyses. Endocrinology 2003; 144: 4773–4782.
Parlevliet ET, Heijboer AC, Schroder-van der Elst JP, Havekes LM, Romijn JA, Pijl H et al. Oxyntomodulin ameliorates glucose intolerance in mice fed a high-fat diet. Am J Physiol Endocrinol Metab 2008; 294: E142–E147.
Sarson DL, Scopinaro N, Bloom SR . Gut hormone changes after jejunoileal (JIB) or biliopancreatic (BPB) bypass surgery for morbid obesity. Int J Obes 1981; 5: 471–480.
Holst JJ, Sorensen TI, Andersen AN, Stadil F, Andersen B, Lauritsen KB et al. Plasma enteroglucagon after jejunoileal bypass with 3:1 or 1:3 jejunoileal ratio. Scand J Gastroenterol 1979; 14: 205–207.
Acknowledgements
This research was funded in part by programme grants from the MRC (G7811974) and Wellcome Trust (072643/Z/03/Z) and by an EU FP6 Integrated Project Grant LSHM-CT-2003-503041. We are also grateful for support from the NIHR Biomedical Research Centre funding scheme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
YLL, HF, JSM, BCTF, JCS, JB and KGM have nothing to declare. MAG and MRD receive royalties from and SRB is an inventor of United Kingdom patent application no. PCT/GB02/04082 and PCT/GB/04/00017. SRB is a consultant from Thiakis, a subsidiary of Wyeth Pharmaceuticals (Pfizer). The company will reveal the structure of the oxyntomodulin analogue and give legitimate investigators the compound for experimental use under the condition that they sign a confidentiality agreement.
Rights and permissions
About this article
Cite this article
Liu, YL., Ford, H., Druce, M. et al. Subcutaneous oxyntomodulin analogue administration reduces body weight in lean and obese rodents. Int J Obes 34, 1715–1725 (2010). https://doi.org/10.1038/ijo.2010.110
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2010.110
Keywords
This article is cited by
-
Selective stimulation of colonic L cells improves metabolic outcomes in mice
Diabetologia (2020)
-
A DPP-IV-resistant triple-acting agonist of GIP, GLP-1 and glucagon receptors with potent glucose-lowering and insulinotropic actions in high-fat-fed mice
Diabetologia (2013)
-
Ghrelin, the proglucagon-derived peptides and peptide YY in nutrient homeostasis
Nature Reviews Gastroenterology & Hepatology (2012)
-
Gut Hormones and Leptin: Impact on Energy Control and Changes After Bariatric Surgery—What the Future Holds
Obesity Surgery (2012)