Abstract
Aim:
The Wnt/β-catenin signaling network offers potential targets to diagnose and uncouple obesity from its metabolic complications. In this study, we investigate the role of the Wnt antagonist, secreted frizzled-related protein 1 (SFRP1), in promoting adipogenesis in vitro and adipose tissue expansion in vivo.
Methods:
We use a combination of human and murine, in vivo and in vitro models of adipogenesis, adipose tissue expansion and obesity-related metabolic syndrome to profile the involvement of SFRP1.
Results:
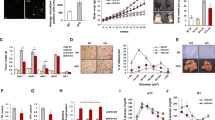
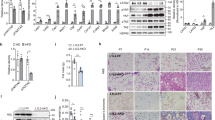
SFRP1 is expressed in both murine and human mature adipocytes. The expression of SFRP1 is induced during in vitro adipogenesis, and SFRP1 is preferentially expressed in mature adipocytes in human adipose tissue. Constitutive ectopic expression of SFRP1 is proadipogenic and inhibits the Wnt/β-catenin signaling pathway. In vivo endogenous levels of adipose SFRP1 are regulated in line with proadipogenic states. However, in longitudinal studies of high-fat-diet-fed mice, we observed a dynamic temporal but biphasic regulation of endogenous SFRP1. In agreement with this profile, we observed that SFRP1 expression in human tissues peaks in patients with mild obesity and gradually falls in morbidly obese subjects.
Conclusions:
Our results suggest that SFRP1 is an endogenous modulator of Wnt/β-catenin signaling and participates in the paracrine regulation of human adipogenesis. The reduced adipose expression of SFRP1 in morbid obesity and its knock-on effect to prevent further adipose tissue expansion may contribute to the development of metabolic complications in these individuals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Despres JP, Lemieux I . Abdominal obesity and metabolic syndrome. Nature 2006; 444: 881–887.
Sethi JK, Vidal-Puig AJ . Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res 2007; 48: 1253–1262.
Virtue S, Vidal-Puig A . It's not how fat you are, it's what you do with it that counts. PLoS Biol 2008; 6: e237.
Tan CY, Vidal-Puig A . Adipose tissue expandability: the metabolic problems of obesity may arise from the inability to become more obese. Biochem Soc Trans 2008; 36: 935–940.
Guilherme A, Virbasius JV, Puri V, Czech MP . Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 2008; 9: 367–377.
Wodarz A, Nusse R . Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol 1998; 14: 59–88.
Logan CY, Nusse R . The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004; 20: 781–810.
Prestwich TC, Macdougald OA . Wnt/beta-catenin signaling in adipogenesis and metabolism. Curr Opin Cell Biol 2007; 19: 612–617.
Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A . Adipogenesis and WNT signalling. Trends Endocrinol Metab 2009; 20: 16–24.
Kennell JA, MacDougald OA . Wnt signaling inhibits adipogenesis through beta-catenin-dependent and -independent mechanisms. J Biol Chem 2005; 280: 24004–24010.
Cawthorn WP, Heyd F, Hegyi K, Sethi JK . Tumour necrosis factor-alpha inhibits adipogenesis via a beta-catenin/TCF4(TCF7L2)-dependent pathway. Cell Death Differ 2007; 14: 1361–1373.
Christodoulides C, Laudes M, Cawthorn WP, Schinner S, Soos M, O’Rahilly S et al. The Wnt antagonist Dickkopf-1 and its receptors are coordinately regulated during early human adipogenesis. J Cell Sci 2006; 119: 2613–2620.
Farmer SR . Transcriptional control of adipocyte formation. Cell Metab 2006; 4: 263–273.
Rosen ED, MacDougald OA . Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 2006; 7: 885–896.
Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati N, Johnson KW et al. Regulation of Wnt signaling during adipogenesis. J Biol Chem 2002; 277: 30998–31004.
Jones SE, Jomary C . Secreted frizzled-related proteins: searching for relationships and patterns. Bioessays 2002; 24: 811–820.
Kawano Y, Kypta R . Secreted antagonists of the Wnt signalling pathway. J Cell Sci 2003; 116: 2627–2634.
Dennis S, Aikawa M, Szeto W, d’Amore PA, Papkoff J . A secreted frizzled related protein, FrzA, selectively associates with Wnt-1 protein and regulates wnt-1 signaling. J Cell Sci 1999; 112 (Pt 21): 3815–3820.
Galli LM, Barnes T, Cheng T, Acosta L, Anglade A, Willert K et al. Differential inhibition of Wnt-3a by Sfrp-1, Sfrp-2, and Sfrp-3. Dev Dyn 2006; 235: 681–690.
Bodine PV, Zhao W, Kharode YP, Bex FJ, Lambert AJ, Goad MB et al. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol 2004; 18: 1222–1237.
Ortega FJ, Mayas D, Moreno-Navarrete JM, Catalan V, Gomez-Ambrosi J, Esteve E et al. The gene expression of the main lipogenic enzymes is downregulated in visceral adipose tissue of obese subjects. Obesity (Silver Spring) 2009; 18: 13–20.
Pietilainen KH, Naukkarinen J, Rissanen A, Saharinen J, Ellonen P, Keranen H et al. Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLoS Med 2008; 5: e51.
Lagathu C, Christodoulides C, Virtue S, Cawthorn WP, Franzin C, Kimber WA et al. Dact1, a nutritionally regulated preadipocyte gene, controls adipogenesis by coordinating the Wnt/beta-catenin signaling network. Diabetes 2009; 58: 609–619.
Medina-Gomez G, Virtue S, Lelliott C, Boiani R, Campbell M, Christodoulides C et al. The link between nutritional status and insulin sensitivity is dependent on the adipocyte-specific peroxisome proliferator-activated receptor-gamma2 isoform. Diabetes 2005; 54: 1706–1716.
Laudes M, Christodoulides C, Sewter C, Rochford JJ, Considine RV, Sethi JK et al. Role of the POZ zinc finger transcription factor FBI-1 in human and murine adipogenesis. J Biol Chem 2004; 279: 11711–11718.
Isakson P, Hammarstedt A, Gustafson B, Smith U . Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes 2009; 58: 1550–1557.
Yang X, Jansson PA, Nagaev I, Jack MM, Carvalho E, Sunnerhagen KS et al. Evidence of impaired adipogenesis in insulin resistance. Biochem Biophys Res Commun 2004; 317: 1045–1051.
Permana PA, Nair S, Lee YH, Luczy-Bachman G, Vozarova De Courten B, Tataranni PA . Subcutaneous abdominal preadipocyte differentiation in vitro inversely correlates with central obesity. Am J Physiol Endocrinol Metab 2004; 286: E958–E962.
Tchoukalova Y, Koutsari C, Jensen M . Committed subcutaneous preadipocytes are reduced in human obesity. Diabetologia 2007; 50: 151–157.
Acknowledgements
We thank the Addenbrooke's hospital surgeons for performing the adipose tissue biopsies. This research is funded by the Biotechnology and Biological Science Research Council (BBSRC), the Medical Research Council (MRC) and the European Union Sixth Framework Programme on Hepatic and Adipose tissue functions in the metabolic syndrome (EU-FP6 HEPADIP) (AV-P and JKS). We also acknowledge the support received from ALFEDIAM and Marie Curie Postdoctoral fellowship (CL), MRC Doctoral Studentships (CC and SV), MRC Career Establishment Award (AV-P) and BBSRC David Phillips Fellowship (JKS).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Lagathu, C., Christodoulides, C., Tan, C. et al. Secreted frizzled-related protein 1 regulates adipose tissue expansion and is dysregulated in severe obesity. Int J Obes 34, 1695–1705 (2010). https://doi.org/10.1038/ijo.2010.107
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2010.107
Keywords
This article is cited by
-
Profiling of N6-methyladenosine methylation in porcine longissimus dorsi muscle and unravelling the hub gene ADIPOQ promotes adipogenesis in an m6A-YTHDF1–dependent manner
Journal of Animal Science and Biotechnology (2023)
-
The size of human subcutaneous adipocytes, but not adiposity, is associated with inflammation, endoplasmic reticulum stress, and insulin resistance markers
Molecular Biology Reports (2023)
-
Pituitary Adenylate Cyclase-activating Polypeptide (PACAP) -derived Peptide MPAPO Stimulates Adipogenic Differentiation by Regulating the Early Stage of Adipogenesis and ERK Signaling Pathway
Stem Cell Reviews and Reports (2023)
-
Metabolic Messengers: tumour necrosis factor
Nature Metabolism (2021)
-
Methylation of secreted frizzled-related protein 1 (SFRP1) promoter downregulates Wnt/β-catenin activity in keloids
Journal of Molecular Histology (2018)