Abstract
Context:
Zinc-α2-glycoprotein (ZAG) was found to influence lipolysis in adipose tissue and has recently been proposed as a candidate factor in the regulation of body weight.
Objective:
To elucidate the association of serum ZAG level with body weight and percentage of body fat in normal, obese subjects and high-fat diet (HFD)-induced obese mice.
Design:
The relationship between serum ZAG and obesity-related parameters was studied in 44 human subjects and 36 mice fed standard food and HFD. Furthermore, the effects of ZAG overexpression on adipose tissue of mice was also evaluated by using a liposome transfection method.
Results:
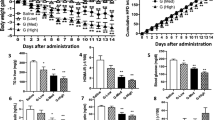
Serum ZAG level was significantly lower in obese patients and obese mice in comparison to that in people and mice with normal weight. The further statistical analysis demonstrated that ZAG level was negatively correlated with body weight (r=−0.62, P<0.001), body mass index (r=−0.64, P<0.001), waist circumference(r=−0.68, P<0.001), hip circumference (r=−0.60, P<0.001), percentage of body fat (r=−0.52, P=0.03) and fat mass(r=−0.59, P=0.01) in human subjects after adjustment for age and sex. Furthermore, ZAG overexpression in mice reduced body weight and the percentage of epididymal fat. The decreased FAS, ACC1 and DGAT mRNA and the increased HSL mRNA were also observed in epididymal adipose tissue in ZAG overexpression mice.
Conclusion:
ZAG is closely linked to obesity. Serum ZAG level is inversely associated with body weight and percentage of body fat. The action of ZAG is associated with downregulated lipogenic enzymes and upregulated lipolytic enzyme expressions in adipose tissue of mice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ahima RS . Adipose tissue as an endocrine organ. Obesity (Silver Spring) 2006; 14 (Suppl 5): 242S–249S.
Burgi W, Schmid K . Preparation and properties of Zn-alpha 2-glycoprotein of normal human plasma. J Biol Chem 1961; 236: 1066–1074.
Poortmans JR, Schmid K . The level of Zn-alpha 2-glycoprotein in normal human body fluids and kidney extract. J Lab Clin Med 1968; 71: 807–811.
Bondar OP, Barnidge DR, Klee EW, Davis BJ, Klee GG . LC-MS/MS quantification of Zn-alpha-2 glycoprotein: a potential serum biomarker for prostate cancer. Clin Chem 2007; 53: 673–678.
Stejskal D, Karpisek M, Reutova H, Stejskal P, Kotolova H, Kollar P . Determination of serum zinc-alpha-2-glycoprotein in patients with metabolic syndrome by a new ELISA. Clin Biochem 2008; 41: 313–316.
Hirai K, Hussey HJ, Barber MD, Price SA, Tisdale MJ . Biological evaluation of a lipid-mobilizing factor isolated from the urine of cancer patients. Cancer Res 1998; 58: 2359–2365.
Hale LP, Price DT, Sanchez LM, Demark-Wahnefried W, Madden JF . Zinc alpha-2-glycoprotein is expressed by malignant prostatic epithelium and may serve as a potential serum marker for prostate cancer. Clin Cancer Res 2001; 7: 846–853.
Russell ST, Zimmerman TP, Domin BA, Tisdale MJ . Induction of lipolysis in vitro and loss of body fat in vivo by zinc-alpha-2-glycoprotein. Biochim Biophys Acta 2004; 1636: 59–68.
Bing C, Bao Y, Jenkins J, Sanders P, Manieri M, Cinti S et al. Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed in adipocytes and is up-regulated in mice with cancer cachexia. Proc Natl Acad Sci USA 2004; 101: 2500–2505.
Bao Y, Bing C, Hunter L, Jenkins JR, Wabitsch M, Trayhurn P . Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed and secreted by human (SGBS) adipocytes. FEBS Lett 2005; 579: 41–47.
Berndt J, Kovacs P, Ruschke K, Kloting N, Fasshauer M, Schon MR et al. Fatty acid synthase gene expression in human adipose tissue: association with obesity and type 2 diabetes. Diabetologia 2007; 50: 1472–1480.
Diraison F, Dusserre E, Vidal H, Sothier M, Beylot M . Increased hepatic lipogenesis but decreased expression of lipogenic gene in adipose tissue in human obesity. Am J Physiol Endocrinol Metab 2002; 282: E46–E51.
Boizard M, Le Liepvre X, Lemarchand P, Foufelle F, Ferre P, Dugail I . Obesity-related overexpression of fatty-acid synthase gene in adipose tissue involves sterol regulatory element-binding protein transcription factors. J Biol Chem 1998; 273: 29164–29171.
Rolland V, Liepvre XL, Jump DB, Lavau M, Dugail I . A GC-rich region containing Sp1 and Sp1-like binding sites is a crucial regulatory motif for fatty acid synthase gene promoter activity in adipocytes. Implication In the overactivity of FAS promoter in obese Zucker rats. J Biol Chem 1996; 271: 21297–21302.
Chen HC, Stone SJ, Zhou P, Buhman KK, Farese Jr RV . Dissociation of obesity and impaired glucose disposal in mice overexpressing acyl coenzyme a:Diacylglycerol acyltransferase 1 in white adipose tissue. Diabetes 2002; 51: 3189–3195.
Sztalryd C, Komaromy MC, Kraemer FB . Overexpression of hormone-sensitive lipase prevents triglyceride accumulation in adipocytes. J Clin Invest 1995; 95: 2652–2661.
Jocken JW, Langin D, Smit E, Saris WH, Valle C, Hul GB et al. Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. J Clin Endocrinol Metab 2007; 92: 2292–2299.
Carlsson E, Johansson LE, Strom K, Hoffstedt J, Groop L, Holm C et al. The hormone-sensitive lipase C-60G promoter polymorphism is associated with increased waist circumference in normal-weight subjects. Int J Obes (Lond) 2006; 30: 1442–1448.
Gong FY, Shi YF, Deng JY . The regulatory mechanism by which interleukin-6 stimulates GH-gene expression in rat GH3 cells. J Endocrinol 2006; 190: 397–406.
Gong FY, Deng JY, Shi YF . IFN-gamma increases the hGH gene promoter activity in rat GH3 cells. Horm Res 2003; 60: 14–20.
Gong FY, Deng JY, Shi YF . Stimulatory effect of interleukin-1beta on growth hormone gene expression and growth hormone release from rat GH3 cells. Neuroendocrinology 2005; 81: 217–228.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408.
Todorov PT, McDevitt TM, Meyer DJ, Ueyama H, Ohkubo I, Tisdale MJ . Purification and characterization of a tumor lipid-mobilizing factor. Cancer Res 1998; 58: 2353–2358.
Kamoshida S, Watanabe K, Suzuki M, Mizutani Y, Sakamoto K, Sugimoto Y et al. Expression of cancer cachexia-related factors in human cancer xenografts: An immunohistochemical analysis. Biomed Res 2006; 27: 275–281.
Groundwater P, Beck SA, Barton C, Adamson C, Ferrier IN, Tisdale MJ . Alteration of serum and urinary lipolytic activity with weight loss in cachectic cancer patients. Br J Cancer 1990; 62: 816–821.
Marrades MP, Martinez JA, Moreno-Aliaga MJ . ZAG, a lipid mobilizing adipokine, is downregulated in human obesity. J Physiol Biochem 2008; 64: 61–66.
Dahlman I, Kaaman M, Olsson T, Tan GD, Bickerton AS, Wahlen K et al. A unique role of monocyte chemoattractant protein 1 among chemokines in adipose tissue of obese subjects. J Clin Endocrinol Metab 2005; 90: 5834–5840.
Rolli V, Radosavljevic M, Astier V, Macquin C, Castan-Laurell I, Visentin V et al. Lipolysis is altered in MHC class I zinc-alpha(2)-glycoprotein deficient mice. FEBS Lett 2007; 581: 394–400.
Russell ST, Tisdale MJ . Effect of a tumour-derived lipid-mobilising factor on glucose and lipid metabolism in vivo. Br J Cancer 2002; 87: 580–584.
Acknowledgements
This research was supported by the national natural science foundation of China (No.: 30540036 and 30771026)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gong, FY., Zhang, SJ., Deng, JY. et al. Zinc-α2-glycoprotein is involved in regulation of body weight through inhibition of lipogenic enzymes in adipose tissue. Int J Obes 33, 1023–1030 (2009). https://doi.org/10.1038/ijo.2009.141
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2009.141
Keywords
This article is cited by
-
Associations of zinc-α-2-glycoprotein with metabolic syndrome and its components among adult Arabs
Scientific Reports (2022)
-
Role of Zinc in Zinc-α2-Glycoprotein Metabolism in Obesity: a Review of Literature
Biological Trace Element Research (2020)
-
Expression and Function of Zinc-α2-Glycoprotein
Neuroscience Bulletin (2019)
-
Effect of prenatal zinc supplementation on adipose tissue-derived hormones and neonatal weight, height and head circumference in women with impaired glucose tolerance test: randomized clinical controlled trial
International Journal of Diabetes in Developing Countries (2019)
-
Hydroxysafflor yellow A (HYSA) inhibited the proliferation and differentiation of 3T3-L1 preadipocytes
Cytotechnology (2015)