Abstract
Objective:
To assess the association between fetal macrosomia and adolescent obesity.
Design:
Longitudinal cohort study of the association between macrosomia and adolescent obesity.
Subjects:
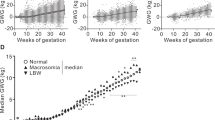
Between 1 October 2005 and 1 February 2007, a follow-up study of live-born infants born in 1993–1995 in Wuxi, a suburban area of Shanghai, was conducted. Subjects with birth weight > 4000 g were selected as the exposed. For each exposed subject, one subject with a birth weight of 2500–4000 g, matched by year of birth, sex of infant, and type of institute at birth, was chosen as non-exposed. Clinical data were collected by structured interview and physical examination. Obesity was defined as body mass index (weight (kg)/height (m2)) higher than the sex-age-specific criteria by the working group on obesity in China. Distribution of baseline characteristics and adolescent obesity rate between the exposed and non-exposed groups was compared.
Results:
A total of 1435 pairs of exposed and non-exposed subjects were included in the final analysis. No major difference in baseline characteristics (other than birth weight) was found between the exposed and non-exposed groups. Obesity rate was significantly higher in the exposed group (2.9%) than in the non-exposed group (1.6%). Adolescent obesity rates were 1.4, 1.9, 2.6, and 5.6%, respectively, in study subjects with a birth weight of 2500–3499, 3500–3999, 4000–4499, and ⩾4500 g. The association between birth weight and adolescent obesity remained essentially the same when mother's demographic and anthropometric factors, breast feeding, and adolescent life-style factors were adjusted.
Conclusion:
Compared with infants of normal birth weight, infants with birth weight >4000 g, especially those >4500 g, are at increased risk of adolescent obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Flegal KM, Carroll MD, Ogden CL, Johnson CL . Prevalence trends in obesity among US adults, 1999-2000. JAMA 2002; 288: 1723–1727.
Ogden CL, Flegal KM, Caroll MD, Johnson CL . Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA 2002; 288: 1728–1732.
WHO. Obesity: Preventing and Managing the Global Epidemic—Report of a WHO Consultation on Obesity. WHO Technical Report Series (no. 894) World Health Organization: Geneva, Switzerland, 2000.
WHO. Life in the 21st Century—A Vision for All. The World Health Rep World Health Org: Geneva, Switzerland, 1998.
McCann J . Obesity, cancer links prompt new recommendations. J Natl Cancer Inst 2001; 93: 901–902.
Statistics Canada. Canadian Community Health Survey: A First Look Cat. No. 11-001E 2002.
Tremblay MS, Willms JD . Secular trends in the body mass index of Canadian children. CMAJ 2000; 163: 1429–1433.
Seidell JC . Obesity, insulin resistance and diabetes-a worldwide epidemic. Br J Nutr 2000; 83: S5–S8.
Alberman E . Are our babies becoming bigger? J R Soc Med 1991; 84: 257–260.
Arbuckle TE, Sherman GJ . An analysis of birth weight by gestational age in Canada. Can Med Assoc J 1989; 140: 157–165.
Institute of Medicine. Nutrition During Pregnancy. National Academy Press: Washington, DC, 1990. pp 1–13 52-56.
Oja H, Koiranen M, Rantakallio P . Fitting mixture models to birth weight data: a case study. Biometrics 1991; 47: 883–897.
Power C . National trends in birth weight: implications for future adult disease. BMJ 1994; 308: 1270–1271.
Singhal PK, Paul VK, Deorari AK, Singh M, Sundaram KR . Changing trends in intrauterine growth curves. Indian Pediatr 1991; 28: 281–283.
Wen SW, Kramer MS, Yang H, Platt R, Demissie K, Joseph KS et al. Secular trends in fetal growth in Canada, 1981–1997. Perinat Pediatr Epidemiol 2003; 17: 347–354.
Westerway SC, Keogh J, Heard R, Morris J . Incidence of fetal macrosomia and birth complications in Chinese immigrant women. Aust N Z J Obstet Gynaecol 2003; 43: 46–49.
Zhao X, Dai ZhY . Changing trend of neonatal birth weight over the past ten years. Shang Hai Yi Xue 2001; 24: 370–372.
Zhu JM, Zhou CX, Yao CF . The change of the birth weight of newborn of the mode of delivery in Xuzhao in the last decade. Zhong Guo Fu Chan Ke Lin Chuang Za Zhi 2004; 5: 420–422.
Liu ShJ, Yao LH, Chen YQ, Liu Z, Sun M . Study on the trend of changes in fetal macrosomia in Yantai during the past 30 years. Zhong Hua Fu Chan Ke Xue Za Zhi 2002; 37: 469–471.
Ji CY, Working group on obesity in China. Report on childhood obesity in China (4) prevalence and trends of overweight and obesity in Chinese urban school-age children and adolescents, 1985-2000. Biomed Environ Sci 2007; 20: 1–10.
Sorensen HT, Sabroe S, Rothman KJ, Gillman M, Fischer P, Sorensen TI . Relation between weight and length at birth and body mass index in young adulthood. BMJ 1997; 315: 1137.
Parsons TJ, Power C, Manor O . Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: longitudinal study. BMJ 2001; 323: 1331–1335.
Pietilainen HKH, Kaprio J, Rasanen M, Winter T, Rissanen A, Rose RJ . Tracking of body size from birth to late adolescence: contributions of birth length, birth weight, duration of gestation, parents' body size, and twenship. Am J Epidemiol 2001; 154: 21–29.
Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA . Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics 2003; 111: e221–e226.
Mikulandra F, Grguriæ J, Banoviæ I, Perisa M, Zakanj Z . The effect of high birth weight (4000 g or more) on the weight and height of adult men and women. Coll Antropol 2000; 24: 133–136.
Stettler N, Tershakovec AM, Zemel BS, Leonard MB, Boston RC, Katz SH et al. Early risk factors for increased adiposity. Am J Clin Nutr 2000; 72: 378–383.
Allison DB, Paultre F, Heymsfield SB, Pi-Sunyer FX . Is the intra-uterine period really a critical period for the development of adiposity? Int J Obes Relat Metab Disord 1995; 19: 397–402.
Berry RJ, Li Z, Erickson JD, Li S, Moore CA, Wang H et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative project for Neural Tube Defect Prevention. N Engl J Med 1999; 341: 1485–1490.
del Río-Navarro BE, Velázquez-Monroy O, Sánchez-Castillo CP, Lara-Esqueda A, Berber A, Fanghänel G et al. The high prevalence of overweight and obesity in Mexican children. Obes Res 2004; 12: 215–223.
Sibai AM, Hwalla N, Adra N, Rahal B . Prevalence and covariates of obesity in Lebanon: findings from the first epidemiological study. Obes Res 2003; 11: 1353–1361.
Discigil G, Tekin N, Soylemez A . Obesity in Turkish children and adolescents: prevalence and non-nutritional correlates in an urban sample. Child Care Health Dev 2009; 35: 153–158.
Eriksson J, Forsen T, Osmond C, Barker D . Obesity from cradle to grave. Int J Obes Relat Metab Disord 2003; 27: 722–727.
Kramer MS, Barr RG, Leduc DG, Boisjoly C, McVey-White L, Pless IB . Determinants of weight and adiposity in the first year of life. Pediatrics 1985; 106: 10–14.
Eriksson JG, Forsen T, Tuomilehto J, Jaddoe VW, Osmond C, Barker DJ . Effects of size at birth and childhood growth on the insulin resistance syndrome in elderly individuals. Diabetologia 2002; 45: 342–348.
Pettitt DJ, Jovanovic L . Birth weight as a predictor of type 2 diabetes mellitus: the U-shaped curve. Curr Diab Rep 2001; 1: 78–81.
Wei JN, Sung FC, Li CY, Chang CH, Lin RS, Chiang CC et al. Low birth weight and high birth weight infants are both at an increased risk to have type 2 diabetes among school children in taiwan. Diabetes Care 2003; 26: 343–348.
Hales CN, Barker DJ . The thrifty phenotype hypothesis. Br Med Bull 2001; 60: 5–20.
Cnattingius S, Bergstrom R, Lipworth L, Kramer MS . Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med 1998; 338: 147–152.
Kramer MS, Platt RW, Wen SW, Joseph KS, Allen A, Abrahamowicz M et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics 2001; 108: E35.
Skjaerven R, Gjessing HK, Bakketeig LS . New standards for birth weight by gestational age using family data. Am J Obstet Gynecol 2000; 183: 689–696.
Al-Qaoud N, Prakash P . Breastfeeding and obesity among Kuwaiti preschool children. Med Princ Pract 2009; 18: 111–117.
Kruger R, Kruger HS, Macintyre UE . The determinants of overweight and obesity among 10- to 15-year-old schoolchildren in the North West Province, South Africa—the THUSA BANA (transition and health during urbanisation of South Africans; BANA, children) study. Public Health Nutr 2006; 9: 351–358n.
Acknowledgements
This study was funded by Canadian Diabetes Association (CDA 1586). Dr Wen is a recipient of the Ontario Women's Health Council-Institute of Gender and Health of Canadian Institutes for Health Research (CIHR) Mid Career Award. Dr Sigal is a Canada Research Chair. Dr Walker is a new investigator of CIHR. We thank all study participants, physicians, nurses, and research staff in Shanghai and Wuxi for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Gao, E., Wu, J. et al. Fetal macrosomia and adolescence obesity: results from a longitudinal cohort study. Int J Obes 33, 923–928 (2009). https://doi.org/10.1038/ijo.2009.131
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2009.131
Keywords
This article is cited by
-
Differential effect of pre-pregnancy low BMI on fetal macrosomia: a population-based cohort study
BMC Medicine (2021)
-
Continuity of midwifery care and gestational weight gain in obese women: a randomised controlled trial
BMC Public Health (2011)
-
Risk factors of obesity in preschool children in an urban area in China
European Journal of Pediatrics (2011)