Abstract
Objective:
Common diseases often have an inflammatory component reflected by associated markers such as serum C-reactive protein (CRP) levels. Circulating CRP levels have also been associated with adipose tissue as well as with specific CRP genotypes. We examined the interaction between measures of body mass index (BMI), waist circumference and fat percent (total fat measured by bioimpedance) with genotypes of the CRP gene in the determination of CRP levels.
Methods:
The first 2296 participants (mean age 76±6 years, 42% men) in the Age, Gene/Environment Susceptibility-Reykjavik Study, a multidisciplinary epidemiological study to determine risk factors in aging, were genotyped for 10 single nucleotide polymorphisms (SNPs) in the CRP gene. General linear models with age and terms for interaction of CRP genotypes with BMI, waist circumference and percent fat were used to evaluate the association of genotypes to CRP levels (high-sensitivity method, range 0–10 mg l−1) in men and women separately.
Results:
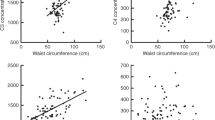
We focused on the SNP rs1205 that represents the allele that captures the strongest effects of the gene on CRP levels. Carriers of the rs1205 G allele had significantly higher CRP levels than noncarriers in a dose-dependent manner. Compared to the AA genotype, the slope of the increase in CRP with increasing BMI (P=0.045) and waist circumference (P=0.014) was different for the G allele carriers and of similar magnitude in both men and women. The rs1205 interactions were not significant for fat mass percent, suggesting a possible association with fat localization.
Conclusions:
This study further illuminates the known association between measures of adiposity and CRP levels and is shown to be dependent on variation in the rs1205 SNP of the CRP gene. The correlated increase in CRP levels with adiposity is accentuated by presence of the G allele.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Finch CE, Morgan T . Systemic inflammation, infection, ApoE alleles, and Alzheimer disease: a position paper. Curr Alzheimer Res 2007; 4: 185–189.
Zaciragic A, Lepara O, Valjevac A, Arslanagic S, Fajkic A, Hadzovic-Dzuvo A et al. Elevated serum C-reactive protein concentration in Bosnian patients with probable Alzheimer's disease. J Alzheimers Dis 2007; 12: 151–156.
Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004; 350: 1387–1397.
Lange LA, Carlsson CS, Hindorff LA, Lange EM, Walston J, Durda JP et al. Association of polymorphisms in the CRP gene with circulating C-reactive protein levels and cardiovascular events. JAMA 2006; 296: 2703–2711.
Kolz M, Koenig W, Müller M, Andreani M, Greven S, Illig T et al. DNA variants, plasma levels and variability of C-reactive protein in myocardial infarction survivors: results from the AIRGENE study. Eur Heart J 2008; 29: 1250–1258.
Hage FG, Szalai AJ . C-reactive protein gene polymorphisms, C-reactive protein blood levels, and cardiovascular disease risk. J Am Coll Cardiol 2007; 50: 1115–1122.
Sattar N, Murray HM, McConnachie A, Blauw GJ, Bollen EL, Buckley BM et al. C-reactive protein and prediction of coronary heart disease and global vascular events in the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER). Circulation 2007; 115: 981–999.
Dehghan A, Kardys I, de Maat MP, Uitterlinden AG, Sijbrands EJ, Bootsma AH et al. Genetic variation, C-reactive protein levels, and incidence of diabetes. Diabetes 2007; 56: 872–878.
Freeman DJ, Nome J, Caslake MJ, Gaw A, Ford I, Lowe GD et al. C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes 2002; 51: 1596–1600.
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM . C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001; 286: 327–334.
Kushner I, Jiang SL, Zhang D, Lozanski G, Samols D . Do post-transcriptional mechanisms participate in induction of C-reactive protein and serum amyloid A by IL-6 and IL-1? Ann NY Acad Sci 1995; 762: 102–107.
Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW . C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol 1999; 19: 972–978.
Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 2006; 17: 4–12.
Anty R, Bekri S, Luciani N, Saint-Paul MC, Dahman M, Iannelli A et al. The inflammatory C-reactive protein is increased in both liver and adipose tissue in severely obese patients independently from metabolic syndrome, type 2 diabetes, and NASH. Am J Gastroenterol 2006; 101: 1824–1833.
Memoli B, Procino A, Calabrò P, Esposito P, Grandaliano G, Pertosa G et al. Inflammation may modulate IL-6 and C-reactive protein gene expression in the adipose tissue: the role of IL-6 cell membrane receptor. Am J Physiol Endocrinol Metab 2007; 293: E1030–E1035.
Forouhi NG, Sattar N, McKeigue PM . Relation of C-reactive protein to body fat distribution and features of the metabolic syndrome in Europeans and South Asians. Int J Obes Relat Metab Disord 2001; 9: 1327–1331.
Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB . Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999; 282: 2131–2135.
Fried SK, Bunkin DA, Greenberg AS . Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab 1998; 83: 847–850.
Ridker PM, Pare G, Parker A, Zee RY, Danik JS, Buring JE et al. Loci related to metabolic-syndrome pathways including LEPR, HNF1A, IL6R, and GCKR associate with plasma C-reactive protein: the Women's Genome Health Study. Am J Hum Genet 2008; 82: 1185–1192.
Carlson CS, Lee PK, Tracy RP, Stephen M, Schwartz SM, Liu K et al. Polymorphisms within the C-reactive protein (CRP) promoter region are associated with plasma CRP levels. Am J Hum Genet 2005; 77: 64–77.
Crawford DC, Qin X, Smith JD, Shephard C, Wong M, Witrak L et al. Genetic variation is associated with C-reactive protein levels in the Third National Health and Nutrition Examination Survey. Circulation 2006; 114: 2458–2465.
Kathiresan S, Larson MG, Vasan RS, Guo CY, Gona P, Keaney Jr JF et al. Contribution of clinical correlates and 13 C-reactive protein gene polymorphisms to interindividual variability in serum C-reactive protein level. Circulation 2006; 113: 1415–1423.
Balistreri CR, Vasto S, Listì F, Grimaldi MP, Lio D, Colonna-Romano G et al. Association between+1059G/C CRP polymorphism and acute myocardial infarction in a cohort of patients from Sicily: a pilot study. Ann NY Acad Sci 2006; 1067: 276–281.
Pai JK, Mukamal KJ, Rexrode KM, Rimm EB . C-reactive protein (CRP) gene polymorphisms, CRP levels, and risk of incident coronary heart disease in two nested case–control studies. PLoS ONE 2008; 3: e1395.
Wang Q, Hunt SC, Xu Q, Chen YE, Province MA, Eckfeldt JH et al. Association study of CRP gene polymorphisms with serum CRP level and cardiovascular risk in the NHLBI Family Heart Study. Am J Physiol Heart Circ Physiol 2006; 291: H2752–H2757.
Kardys I, Knetsch AM, Bleumink GS, Deckers JW, Hofman A, Stricker BH et al. C-reactive protein and risk of heart failure. The Rotterdam Study. Am Heart J 2006; 152: 514–520.
Jónsdóttir LS, Sigfusson N, Sigvaldason H, Thorgeirsson G . Incidence and prevalence of recognised and unrecognised myocardial infarction in women. The Reykjavik Study. Eur Heart J 1998; 19: 1011–1018.
Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol 2007; 165: 1076–1087.
Lemieux I, Pascot A, Prud'homme D, Alméras N, Bogaty P, Nadeau A et al. Elevated C-reactive protein another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol 2001; 21: 961–967.
Piché ME, Lapointe A, Weisnagel SJ, Corneau L, Nadeau A, Bergeron J et al. Regional body fat distribution and metabolic profile in postmenopausal women. Metabolism 2008; 57: 1101–1107.
Piché ME, Lemieux S, Weisnagel SJ, Corneau L, Nadeau A, Bergeron J . Relation of high-sensitivity C-reactive protein, interleukin-6, tumor necrosis factor-alpha, and fibrinogen to abdominal adipose tissue, blood pressure, and cholesterol and triglyceride levels in healthy postmenopausal women. Am J Cardiol 2005; 96: 92–97.
Toniatti C, Demartis A, Monaci P, Nicosia A, Ciliberto G . Synergistic trans-activation of the human C-reactive protein promoter by transcription factor HNF-1 binding at two distinct sites. EMBO J 1990; 9: 4467–4475.
Lozanski G, Jiang SL, Samols D, Kushner I . C-reactive protein and serum amyloid A mRNA stability following induction by cytokines. Cytokine 1996; 8: 534–540.
Brull DJ, Serrano N, Zito F, Jones L, Montgomery HE, Rumley A et al. Human CRP gene polymorphism influences CRP levels: implications for the prediction and pathogenesis of coronary heart disease. Arterioscler Thromb Vasc Biol 2003; 23: 2063–2069.
Danik JS, Ridker PM . Genetic determinants of C-reactive protein. Curr Atheroscler Rep 2007; 9: 195–203.
Zee RY, Germer S, Thomas A, Raji A, Rhees B, Ridker PM et al. C-reactive protein gene variation and type 2 diabetes mellitus: a case–control study. Atherosclerosis 2008; 197: 931–936.
Acknowledgements
This study was funded by National Institutes of Health contract N01-AG-12100, the National Institute on Aging Intramural Research Program, Hjartavernd (the Icelandic Heart Association) and the Althingi (the Icelandic Parliament).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eiriksdottir, G., Smith, A., Aspelund, T. et al. The interaction of adiposity with the CRP gene affects CRP levels: Age, Gene/Environment Susceptibilty-Reykjavik Study. Int J Obes 33, 267–272 (2009). https://doi.org/10.1038/ijo.2008.274
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2008.274
Keywords
This article is cited by
-
Association of − 717 A > G (rs2794521) CRP polymorphism with high cardiovascular risk by C-reactive protein in systemic lupus erythematosus patients
Clinical Rheumatology (2023)
-
C-reactive protein gene rs1205 polymorphism is associated with low-grade chronic inflammation in postmenopausal women
Women's Midlife Health (2020)
-
C-reactive protein gene variants: independent association with late-life depression and circulating protein levels
Translational Psychiatry (2015)
-
Interactions of genetic and non-genetic factors on plasma hs-CRP concentration in a Korean community-based cohort study
Genes & Genomics (2015)
-
Genetic risk score and adiposity interact to influence triglyceride levels in a cohort of Filipino women
Nutrition & Diabetes (2014)