Abstract
Introduction:
Analysis of several biological markers improves the quality and physiologic comprehension of data obtained in epidemiological nutritional studies.
Aim:
To develop a methodology that guarantees the centralized analysis and quality assurance of the most relevant blood parameters from fresh blood samples in adolescents in a European multicenter study.
Materials and methods:
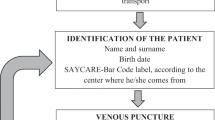
Stability of selected nutrients and biomarkers (vitamins, fatty acids, iron metabolism and immunological parameters) chosen with respect to time and temperature of sample transport and storage was evaluated as part of the pilot study of the Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) project.
Results:
Routine biochemistry and iron status parameters included in the HELENA Cross-Sectional Study (CSS) protocol could be analyzed within 24 h from fresh blood samples without any stability problems (coefficient of variation (CV)<5%, P<0.05). However, stability tests for lymphocyte subpopulations, vitamin C and fatty acids showed that they are very unstable at room temperature without any treatment. Therefore, a special handling for these samples was developed. Vitamin C was stabilized with metaphosphoric acid and transported under cooled conditions (CV 4.4%, recovery rate >93%, P>0.05). According to the results, a specific methodology and transport system were developed to collect blood samples at schools in 10 European cities and to send them to the centralized laboratory (IEL, Bonn, Germany). To guarantee good clinical practice, the field workers were instructed in a training workshop and a manual of operation was developed.
Conclusion:
The handling and transport system for fresh blood samples developed for the European multicenter study HELENA is adequate for the final part of the HELENA-CSS and will provide, for the first time, reference values for several biological markers in European adolescents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kaaks RJ . Biochemical markers as additional measurements in studies of the accuracy of dietary questionnaire measurements: conceptual issues. Am J Clin Nutr Suppl 1997; 65: 1232–1239.
Allison S . Malnutrition, disease, and outcome. Nutrition 2000; 16: 590–593.
Pinhas-Hamiel O, Newfield RS, Koren I, Agmon A, Lilos P, Philip M . Greater prevalence of iron deficiency in overweight and obese children and adolescents. Int J Obes 2003; 27: 416–418.
Nead KG, Halterman JS, Kaczorowski JM, Auinger P, Weitzman M . Overweight children and adolescents: a risk group for iron deficiency. Pediatrics 2004; 114: 104–108.
Pinhas-Hamiel O, Doron-Panush N, Reichman B, Nitzan-Kaluski D, Shalitin S, Geva-Lerner L . Obese Children and Adolescents. A risk group for low vitamin B12 concentration. Arch Pediatr Adolesc Med 2006; 160: 933–936.
Decsi T, Molnar D, Koletzko B . Reduced plasma concentration of alpha-tocopherol and beta-carotene in obese boys. J Pediatr 1997; 130: 653–655.
Molnar D, Decsi T, Koletzko B . Reduced antioxidant status in obese children with multimetabolic syndrome. Int J Obes Relat Metab Disord 2004; 28: 1197–1202.
Thurnham DI, Mburu ASW, Mwaniki DL, De Wagt A . Micronutrients in childhood and the influence of subclinical inflammation. Proc Nutr Soc 2005; 64: 502–509.
Kersting M, Breidenassel C, Sichert-Hellert W, Koeppen E, Gedrich K, Rieken K et al., im Namen der HELENA Studiengruppe. HELENA: healthy lifestyle in Europe by nutrition in adolescence. Ernährung Wissenschaft und Praxis 2007; 1: 17–22.
de Henauw S, Gottrand F, De Bourdeaudhuij I, Gonzalez-Gross M, Leclercq C, Kafatos A et al., on behalf of the HELENA Study Group Nutritional status and lifestyle of adolescents in a public health perspective. The HELENA project—healthy lifestyle in Europe by nutrition in adolescence. J Public Health 2007; 3: 187–197.
Moreno LA, González-Gross M, Kersting M, Molnár D, de Henauw S, Beghin L et al., on behalf of the HELENA Study Group. Assessing, understanding and modifying nutritional status, eating habits and physical activity in European adolescents. The HELENA Study. Public Health Nutr 2007; 6: 1–12.
Al-Delaimy WK, Jansen EH, Peeters PH, van der Laan JD, van Noord PA, Boshuizen HC et al. Reliability of biomarkers of iron status, blood lipids, oxidative stress, vitamin D, C-reactive protein and fructosamine in two Dutch cohorts. Biomarkers 2006; 11: 370–382.
The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. www.ich.org.Visited 23 November 2006.
IATA DANGEROUS GOODS, 2005. www.iata.org. Visited 15 June 2006.
European parliament directive 2001/20/CE of the European parliament and council of 4 April 2001. Official Journal 2001; L121: 34–44.
Bland JM, Altman DG . Measuring agreement in method comparison studies. Stat Methods Med Res 1999; 8: 135–160.
Erhardt J, Biesalski HK . Diagnosis of nutritional anemia—laboratory assessment of iron status. The GuideBook—Nutritional Anemia, Sight and Light (Chapter 4), 2007.
Muhl H, Nold M, Chang JH, Frank S, Eberhardt W, Pfeilschifter J . Expression and release of chemokines associated with apoptotic cell death in human promonocytic U937 cells and peripheral blood mononuclear cells. Eur J Immunol 1999; 29: 3225–3235.
Al-Tahan J, González-Gross M, Pietrzik K . Vitamin status and intake in European adolescents. A review of the literature. Nutr Hosp 2006; 21: 452–465.
Acknowledgements
The HELENA Study was carried out with the financial support of the European Community Sixth RTD Framework Programme (Contract FOOD-CT-2005-007034). The content of this article reflects only the authors’ views, and the European Community is not liable for any use that may be made of the information contained therein. Many thanks to Christel Bierschbach, Adelheid Schuch, Petra Pickert, Anke Carstensen for their contribution to laboratory work, and to Marie-Adélaïde Bout for printing LRF and managing the automatic record of 2D-code.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Conflict of interest
The authors state no conflict of interest.
Rights and permissions
About this article
Cite this article
González-Gross, M., Breidenassel, C., Gómez-Martínez, S. et al. Sampling and processing of fresh blood samples within a European multicenter nutritional study: evaluation of biomarker stability during transport and storage. Int J Obes 32 (Suppl 5), S66–S75 (2008). https://doi.org/10.1038/ijo.2008.185
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2008.185
Keywords
This article is cited by
-
Association between PTPN1 polymorphisms and obesity-related phenotypes in European adolescents: influence of physical activity
Pediatric Research (2023)
-
Association between adherence to the EAT-Lancet sustainable reference diet and cardiovascular health among European adolescents: the HELENA study
European Journal of Clinical Nutrition (2023)
-
Relative validity of the Planetary Health Diet Index by comparison with usual nutrient intakes, plasma food consumption biomarkers, and adherence to the Mediterranean diet among European adolescents: the HELENA study
European Journal of Nutrition (2023)
-
Interplay of physical activity and genetic variants of the endothelial lipase on cardiovascular disease risk factors
Pediatric Research (2022)
-
Development of a Genetic Risk Score to predict the risk of overweight and obesity in European adolescents from the HELENA study
Scientific Reports (2021)