Abstract
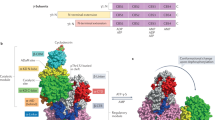
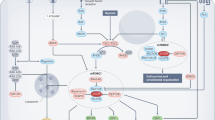
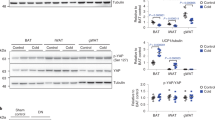
The AMP-activated protein kinase (AMPK) system is a key player in regulating energy balance at both the cellular and whole-body levels, placing it at centre stage in studies of obesity, diabetes and the metabolic syndrome. It is switched on in response to metabolic stresses such as muscle contraction or hypoxia, and modulated by hormones and cytokines affecting whole-body energy balance such as leptin, adiponectin, resistin, ghrelin and cannabinoids. Once activated, it switches on catabolic pathways that generate adenosine triphosphate (ATP), while switching off ATP-consuming anabolic processes. AMPK exists as heterotrimeric complexes comprising a catalytic α-subunit and regulatory β- and γ-subunits. Binding of AMP to the γ-subunit, which is antagonized by high ATP, causes activation of the kinase by promoting phosphorylation at threonine (Thr-172) on the α-subunit by the upstream kinase LKB1, allowing the system to act as a sensor of cellular energy status. In certain cells, AMPK is activated in response to elevation of cytosolic Ca2+ via phosphorylation of Thr-172 by calmodulin-dependent kinase kinase-β (CaMKKβ). Activation of AMPK, either in response to exercise or to pharmacological agents, has considerable potential to reverse the metabolic abnormalities associated with type 2 diabetes and the metabolic syndrome. Two existing classes of antidiabetic drugs, that is, biguanides (for example, metformin) and the thiazolidinediones (for example, rosiglitazone), both act (at least in part) by activation of AMPK. Novel drugs activating AMPK may also have potential for the treatment of obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Hardie DG, Carling D, Sim ATR . The AMP-activated protein kinase—a multisubstrate regulator of lipid metabolism. Trends Biochem Sci 1989; 14: 20–23.
Kahn BB, Alquier T, Carling D, Hardie DG . AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 2005; 1: 15–25.
Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Mäkelä TP et al. Complexes between the LKB1 tumor suppressor, STRADa/b and MO25a/b are upstream kinases in the AMP-activated protein kinase cascade. J Biol 2003; 2: 28.
Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol 2003; 13: 2004–2008.
Alessi DR, Sakamoto K, Bayascas JR . Lkb1-dependent signaling pathways. Annu Rev Biochem 2006; 75: 137–163.
Hardie DG, Salt IP, Hawley SA, Davies SP . AMP-activated protein kinase: an ultrasensitive system for monitoring cellular energy charge. Biochem J 1999; 338: 717–722.
Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2005; 2: 9–19.
Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR et al. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2005; 2: 21–33.
Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA . The Ca2+/calmoldulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem 2005; 280: 29060–29066.
Hudson ER, Pan DA, James J, Lucocq JM, Hawley SA, Green KA et al. A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Current Biol 2003; 13: 861–866.
Jørgensen SB, Nielsen JN, Birk JB, Olsen GS, Viollet B, Andreelli F et al. The alpha2-5′AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes 2004; 53: 3074–3081.
Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA et al. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest 2004; 113: 274–284.
Burwinkel B, Scott JW, Bührer C, van Landeghem FK, Cox GF, Wilson CJ et al. Fatal congenital heart glycogenosis caused by a recurrent activating R531Q mutation in the g2 subunit of AMP-activated protein kinase (PRKAG2), not by phosphorylase kinase deficiency. Am J Hum Genet 2005; 76: 1034–1049.
da Silva Xavier G, Leclerc I, Varadi A, Tsuboi T, Moule SK, Rutter GA . Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem J 2003; 371: 761–774.
Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004; 428: 569–574.
Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR et al. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem 2004; 279: 12005–12008.
Evans AM, Mustard KJ, Wyatt CN, Peers C, Dipp M, Kumar P et al. Does AMP-activated protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to calcium signaling in O2-sensing cells? J Biol Chem 2005; 280: 41504–41511.
Winder WW, Hardie DG . Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol 1996; 270: E299–E304.
Mu J, Brozinick JT, Valladares O, Bucan M, Birnbaum MJ . A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 2001; 7: 1085–1094.
Sakamoto K, McCarthy A, Smith D, Green KA, Hardie GD, Ashworth A et al. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J 2005; 24: 1810–1820.
Zheng D, MacLean PS, Pohnert SC, Knight JB, Olson AL, Winder WW et al. Regulation of muscle GLUT-4 transcription by AMP-activated protein kinase. J Appl Physiol 2001; 91: 1073–1083.
Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ et al. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci USA 2002; 99: 15983–15987.
Kola B, Hubina E, Tucci SA, Kirkham TC, Garcia EA, Mitchell SE et al. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated Protein Kinase. J Biol Chem 2005; 280: 25196–25201.
Stahmann N, Woods A, Carling D, Heller R . Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase beta. Mol Cell Biol 2006; 26: 5933–5945.
Tamás P, Hawley SA, Clarke RG, Mustard KJ, Green K, Hardie DG et al. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med 2006; 203: 1665–1670.
Inoki K, Zhu T, Guan KL . TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003; 115: 577–590.
Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L et al. Activation of AMP-activated protein kinase leads to the phosphorylation of Elongation Factor 2 and an inhibition of protein synthesis. Current Biol 2002; 12: 1419–1423.
Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H . Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun 2001; 287: 562–567.
Lowell BB, Shulman GI . Mitochondrial dysfunction and type 2 diabetes. Science 2005; 307: 384–387.
Hardie DG . AMP-activated protein kinase: a master switch in glucose and lipid metabolism. Rev Endocr Metab Disord 2004; 5: 119–125.
Fryer LG, Parbu-Patel A, Carling D . The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct pathways. J Biol Chem 2002; 277: 25226–25232.
Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001; 108: 1167–1174.
Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R et al. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions. Diabetes 2004; 53: 1052–1059.
Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA . An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem 1995; 270: 12953–12956.
Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K et al. PPARγ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 2001; 50: 2094–2099.
Kubota N, Terauchi Y, Kubota T, Kumagai H, Itoh S, Satoh H et al. Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and -independent pathways. J Biol Chem 2006; 281: 8748–8755.
Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005; 310: 1642–1646.
Dobrzyn P, Dobrzyn A, Miyazaki M, Cohen P, Asilmaz E, Hardie DG et al. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proc Natl Acad Sci USA 2004; 101: 6409–6414.
Matejkova O, Mustard KJ, Sponarova J, Flachs P, Rossmeisl M, Miksik I et al. Possible involvement of AMP-activated protein kinase in obesity resistance induced by respiratory uncoupling in white fat. FEBS Lett 2004; 569: 245–248.
Schrauwen P, Hardie DG, Roorda B, Clapham JC, Abuin A, Thomason-Hughes M et al. Improved glucose homeostasis in mice overexpressing human UCP3: a role for AMP-kinase? Int J Obes Relat Metab Disord 2004; 28: 824–828.
Acknowledgements
Recent studies in the author's laboratory have been funded by Programme Grants from the Wellcome Trust, and by the EXGENESIS Integrated project (LSHM-CT-2004-005272) funded by the European Commission.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
The author states no conflict of interest.
Rights and permissions
About this article
Cite this article
Hardie, D. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes 32 (Suppl 4), S7–S12 (2008). https://doi.org/10.1038/ijo.2008.116
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2008.116
Keywords
This article is cited by
-
AMPK pathway: an emerging target to control diabetes mellitus and its related complications
Journal of Diabetes & Metabolic Disorders (2024)
-
A new AMPK isoform mediates glucose-restriction induced longevity non-cell autonomously by promoting membrane fluidity
Nature Communications (2023)
-
Antioxidant effects of silver nanoparticles obtained by green synthesis from the aqueous extract of Eryngium carlinae on the brain mitochondria of streptozotocin-induced diabetic rats
Journal of Bioenergetics and Biomembranes (2023)
-
The role of miR-143/miR-145 in the development, diagnosis, and treatment of diabetes
Journal of Diabetes & Metabolic Disorders (2023)
-
Resveratrol butyrate esters inhibit lipid biosynthesis in 3T3-L1 cells by AMP-activated protein kinase phosphorylation
Journal of Food Science and Technology (2023)