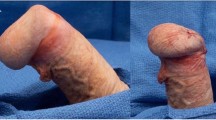
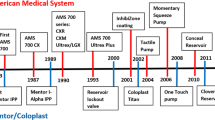
Abstract
Penile prosthesis implantation is the oldest effective treatment for erectile dysfunction. This review examines the past, present and future of penile prosthesis implantation. Advances in prosthetic design and implantation techniques have resulted today in devices that produce nearly normal flaccid and erect states, and have remarkable freedom from mechanical failure. The future of prosthetic design holds promises for even more improvements.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Beheri GE . Surgical treatment of impotence. Plast Reconstr Surg 1966; 38: 92.
Loeffler RA, Sayegh ES . Perforated acrylic implants in management of organic impotence. J Urol 1960; 84: 559.
Stafford-Clark D . The etiology and treatment of impotence. Practitioner 1954; 172: 397.
Scott FB, Bradley WE, Timm GW . Management of erectile impotence: use of implantable inflatable prosthesis. Urology 1973; 2: 80–82.
Brindley GS . Cavernosal alpha-blockade: a new technique for investigating and treating erectile impotence. Br J Psychiatry 1983; 143: 332.
Virag R . Intracavernous injection of papaverine for erectile failure. Letter to the editor. Lancet 1982; 2: 938.
Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA . Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group [see comments] [published erratum appears in N Engl J Med 1998 Jul 2;339(1):59]. N Engl J Med 1998; 338: 1397–1404.
Goodwin WE, Scott WW . Phalloplasty. J Urol 1952; 68: 903.
Lash H . Silicone implant for impotence. J Urol 1968; 100: 709.
Pearman RO . Treatment of organic impotence by implantation of a penile prosthesis. J Urol 1967; 97: 716.
Pearman RO . Insertion of a silastic penile prosthesis for the treatment of organic sexual impotence. J Urol 1972; 107: 802–806.
Beheri GE . Beheri's operation for treatment of impotence-observations on 125 cases. Kasr el Aini J Surg 1960; 1: 390.
Beheri GE . The problem of impotence solved by a new surgical operation. Kasr el Aini J Surg 1960; 1: 50.
Fisher C, Schiavi RC, Edwards A, Davis DM, Reitman M, Fine J . Evaluation of nocturnal penile tumescence in the differential diagnosis of sexual impotence. Arch Gen Psychiatry 1979; 36: 431–437.
Furlow WL . Inflatable penile prosthesis: Mayo clinic experience with 175 patients. Urology 1979; 13: 166–171.
Kabalin JN, Kessler R . Five-year followup of the Scott inflatable penile prosthesis and comparison with semirigid penile prosthesis. J Urol 1988; 140: 1428–1430.
Malloy TR, Wein AJ, Carpiniello VL . Further experience with the inflatable penile prosthesis. J Urol 1979; 122: 478–480.
Merrill DC . Clinical experience with Scott inflatable penile prosthesis in 150 patients. Urology 1983; 22: 371–375.
Montague DK . Experience with semirigid rod and inflatable penile prostheses. J Urol 1983; 129: 967–968.
Small MP . Small–Carrion penile prosthesis: a report on 160 cases and review of the literature. J Urol 1978; 119: 365–368.
Small MP, Carrion HM, Gordon JA . Small-Carrion penile prosthesis: new implant for management of impotence. Urology 1975; 5: 479.
Finney RP . New hinged silicone penile implant. J Urol 1977; 118: 585–587.
Finney RP, Sharpe JR, Sadlowski RW . Finney hinged penile implant: experience with 100 cases. J Urol 1980; 124: 205–207.
Jonas U, Jacobi GH . Silicone-silver penile prosthesis: description, operative approach and results. J Urol 1980; 123: 865–867.
Dorflinger T, Bruskewitz R . AMS malleable penile prosthesis. Urology 1986; 28: 480–485.
Moul JW, McLeod DG . Experience with the AMS 600 malleable penile prosthesis. J Urol 1986; 135: 929–931.
Salama N . Satisfaction with the malleable penile prosthesis among couples from the Middle East—is it different from that reported elsewhere? Int J Impot Res 2004; 16: 175–180.
Huisman TK, Macintyre RC . Mechanical failure of Omniphase penile prosthesis. Urology 1988; 31: 515–516.
Levinson K, Whitehead ED . Omniphase penile prosthesis: delayed bilateral central cable breakage. J Urol 1989; 141: 618–619.
Hrebinko R, Bahnson RR, Schwentker FN, O’Donnell WF . Early experience with the Duraphase penile prosthesis. J Urol 1990; 143: 60–61.
Thompson IM, Rodriguez FR, Zeidman EJ . Experience with Duraphase penile prosthesis: its use as replacement device. Urology 1990; 36: 505–507.
Mulcahy JJ, Krane RJ, Lloyd LK, Edson M, Siroky MB . Duraphase penile prosthesis-results of clinical trials in 63 patients. J Urol 1990; 143: 518–519.
Finney RP . Flexi-flate penile prosthesis. Semin Urol 1986; 4: 244–246.
Stanisic TH, Dean JC . The flexi-flate and flexi-flate II penile prostheses. Urol Clin North Am 1989; 16: 39–49.
Stanisic TH, Dean JC, Donovan JM, Beutler LE . Clinical experience with a self-contained inflatable penile implant: the Flexi-Flate. J Urol 1988; 139: 947–950.
Fishman IJ . Experience with the hydroflex penile prosthesis. Semin Urol 1986; 4: 239–243.
Kabalin JN, Kessler R . Experience with the hydroflex penile prosthesis. J Urol 1989; 141: 58–59.
Mulcahy JJ . The hydroflex self-contained inflatable penile prosthesis: experience with 100 patients. J Urol 1988; 140: 1422–1423.
Anafarta K, Yaman O, Aydos K . Clinical experience with Dynaflex penile prostheses in 120 patients. Urology 1998; 52: 1098–1100.
Merrill DC . Mentor inflatable penile prostheses. Urol Clin North Am 1989; 16: 51–66.
Engel RME, Fein RL . Mentor GFS inflatable prosthesis. Urology 1990; 35: 405–406.
Fein RL . The G. F. S. mark II inflatable penile prosthesis. J Urol 1992; 147: 66–68.
Fallon B, Rosenberg S, Culp DA . Long-term followup in patients with an inflatable penile prosthesis. J Urol 1984; 132: 270–271.
Pereira Arias JG, Escobal Tamayo V, Marana Fernandez MT, Astobieta Odriozola A, Bernuy Malfaz C . [Penile prosthetic implant in the treatment of impotence: our experience]. Arch Esp Urol 1994; 47: 703–708.
Merrill DC . Mentor inflatable penile prosthesis. Urology 1983; 22: 504–505.
Goldstein I, Bertero EB, Kaufman JM, Witten FR, Hubbard JG, Fitch WP et al. Early experience with the first pre-connected 3-piece inflatable penile prosthesis: the Mentor Alpha-1. [see comments]. J Urol 1993; 150: 1814–1818.
Garber BB . Mentor Alpha 1 inflatable penile prosthesis: patient satisfaction and device reliability. Urology 1994; 43: 214–217.
Fathy A, Shamloul R, AbdelRahim A, Zeidan A, El-Dakhly R, Ghanem H . Experience with tube (Promedon) malleable penile implant. Urol Int 2007; 79: 244–247.
Subrini L . [Surgical treatment of virile impotence: intracavernous intubation]. J Urol Nephrol (Paris) 1973; 79: 647–653.
Subrini L . [A biomechanical study of flexible penile implants]. Ann Urol (Paris) 1993; 27: 192–196.
Subrini L . [Flexible penile implants in the restoration of erectile function]. Ann Urol (Paris) 1993; 27: 183–191.
Subrini L . [Flexible penile implants. An experience over 60 cases]. Ann Chir Plast Esthet 1994; 39: 15–26.
Ferguson KH, Cespedes RD . Prospective long-term results and quality-of-life assessment after Dura-II penile prosthesis placement. Urology 2003; 61: 437–441.
Kearse Jr WS, Sago AL, Peretsman SJ, Bolton JO, Holcomb RG, Reddy PK et al. Report of a multicenter clinical evaluation of the Dura-II penile prosthesis. J Urol 1996; 155: 1613–1616.
Levine LA, Estrada CR, Morgentaler A . Mechanical reliability and safety of, and patient satisfaction with the Ambicor inflatable penile prosthesis: results of a 2 center study. J Urol 2001; 166: 932–937.
Lux M, Reyes-Vallejo L, Morgentaler A, Levine LA . Outcomes and satisfaction rates for the redesigned 2-piece penile prosthesis. J Urol 2007; 177: 262–266.
Wilson SK, Henry GD, Delk Jr JR, Cleves MA . The mentor Alpha 1 penile prosthesis with reservoir lock-out valve: effective prevention of auto-inflation with improved capability for ectopic reservoir placement. J Urol 2002; 168: 1475–1478.
Montague DK, Jarow JP, Broderick GA, Dmochowski RR, Heaton JP, Lue TF, et al., Erectile Dysfunction Guideline Update Panel. Chapter 1: the management of erectile dysfunction: an AUA update. J Urol 2005; 174: 230–239.
Deuk Choi Y, Jin Choi Y, Hwan Kim J, Ki Choi H . Mechanical reliability of the AMS 700CXM inflatable penile prosthesis for the treatment of male erectile dysfunction. J Urol 2001; 165: 822–824.
Carson CC, Mulcahy JJ, Govier FE . Efficacy, safety and patient satisfaction outcomes of the AMS 700CX inflatable penile prosthesis: results of a long-term multicenter study. AMS 700CX Study Group. J Urol 2000; 164: 376–380.
Montorsi F, Rigatti P, Carmignani G, Corbu C, Campo B, Ordesi G et al. AMS three-piece inflatable implants for erectile dysfunction: a long-term multi-institutional study in 200 consecutive patients. Eur Urol 2000; 37: 50–55.
Daitch JA, Angermeier KW, Lakin MM, Ingleright BJ, Montague DK . Long-term mechanical reliability of AMS 700 series inflatable penile prostheses: comparison of CX/CXM and Ultrex cylinders. J Urol 1997; 158: 1400–1402.
Dubocq F, Tefilli MV, Gheiler EL, Li H, Dhabuwala CB . Long-term mechanical reliability of multicomponent inflatable penile prosthesis: comparison of device survival. Urology 1998; 52: 277–281.
Milbank AJ, Montague DK, Angermeier KW, Lakin MM, Worley SE . Mechanical failure of the American Medical Systems Ultrex inflatable penile prosthesis: before and after 1993 structural modification. J Urol 2002; 167: 2502–2506.
Goldstein I, Newman L, Baum N, Brooks M, Chaikin L, Goldberg K et al. Safety and efficacy outcome of Mentor Alpha-1 inflatable penile prosthesis implantation for impotence treatment. J Urol 1997; 157: 833–839.
Wilson SK, Cleves MA, Delk II JR . Comparison of mechanical reliability of original and enhanced Mentor Alpha I penile prosthesis. J Urol 1999; 162: 715–718.
Dhar NB, Angermeier KW, Montague DK . Long-term mechanical reliability of AMS 700CX/CXM inflatable penile prosthesis. J Urol 2006; 176: 2599–2601; discussion 2601.
Wilson SK, Delk JR, Salem EA, Cleves MA . Long-term survival of inflatable penile prostheses: single surgical group experience with 2384 first-time implants spanning two decades. J Sex Med 2007; 4: 1074–1079.
Carson III CC . Efficacy of antibiotic impregnation of inflatable penile prostheses in decreasing infection in original implants. J Urol 2004; 171: 1611–1614.
Carson CC . Initial success with AMS 700 series inflatable penile prosthesis with Inhibizone antibiotic surface treatment: a retrospective review of revision cases incidence and comparative results versus non-treated devices. J Urol 2004; 171: S894.
Wolter CE, Hellstrom JG . The hydrophilic-coated penile prosthesis: 1-year experience. J Sex Med 2004; 1: 221–224.
Kelly DA . Penises as variable-volume hydrostatic skeletons. Ann N Y Acad Sci 2007; 1101: 453–463.
Simmons MN, Montague DK . Novel paradigms in penile prosthetic design. AUA Annual Meeting 2007, Vol 177, abstract no. 939, p 311.
Seeman NC . At the crossroads of chemistry, biology, and materials: structural DNA nanotechnology. Chem Biol 2003; 10: 1151–1159.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simmons, M., Montague, D. Penile prosthesis implantation: past, present and future. Int J Impot Res 20, 437–444 (2008). https://doi.org/10.1038/ijir.2008.11
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijir.2008.11
Keywords
This article is cited by
-
Auf der Suche nach einer Kultur- und Technikgeschichte der männlichen Genitalprothetik
Der Urologe (2019)
-
How to Treat Erectile Dysfunction in Men with Diabetes: from Pathophysiology to Treatment
Current Diabetes Reports (2014)
-
Twenty years of IJIR
International Journal of Impotence Research (2008)