Abstract
Obesity is a growing global health concern, with a rapid increase being observed in morbid obesity. Obesity is associated with an increased cardiovascular risk and earlier onset of cardiovascular morbidity. The growing obesity epidemic is a major source of unsustainable health costs and morbidity and mortality because of hypertension, type 2 diabetes mellitus, dyslipidemia, certain cancers and major cardiovascular diseases. Similar to obesity, hypertension is a key unfavorable health metric that has disastrous health implications: currently, hypertension is the leading contributor to global disease burden, and the direct and indirect costs of treating hypertension are exponentially higher. Poor lifestyle characteristics and health metrics often cluster together to create complex and difficult-to-treat phenotypes: excess body mass is such an example, facilitating a cascade of pathophysiological sequelae that create such as a direct obesity–hypertension link, which consequently increases cardiovascular risk. Although some significant issues regarding assessment/management of obesity remain to be addressed and the underlying mechanisms governing these disparate effects of obesity on cardiovascular disease are complex and not completely understood, a variety of factors could have a critical role. Consequently, a comprehensive and exhaustive investigation of this relationship should analyze the pathogenetic factors and pathophysiological mechanisms linking obesity to hypertension as they provide the basis for a rational therapeutic strategy in the aim to fully describe and understand the obesity–hypertension link and discuss strategies to address the potential negative consequences from the perspective of both primordial prevention and treatment for those already impacted by this condition.
Similar content being viewed by others
Introduction
During the past three decades, the worldwide prevalence of obesity has nearly doubled, and the mean body mass index (BMI) has increased worldwide by 0.4 kg m−2 per decade for men and 0.5 kg m−2 per decade for women.1 Although obesity is merely one of the various cardiovascular disease risk factors, it has received a lot of medical attention lately as the prevalence of obesity continues to increase globally.2
Obesity is a growing global health concern, with a rapid increase being observed in morbid obesity. Excess body weight is associated with an increased cardiovascular risk and earlier onset of cardiovascular morbidity.3 It is well established that obesity is associated with activation of both the sympathetic nervous system and the renin–angiotensin system contributing to the emergence of hypertension.4 Epidemiological studies have demonstrated the importance of the level and duration of obesity as risk factors for cardiovascular disease.5 Improving preventive medical care to detect, treat and control of risk factors in overweight and obese patients is therefore of particular importance.6 Smoking, high blood pressure and elevated serum cholesterol levels represent major cardiovascular risk factors7 that are routinely managed in primary care settings. Deficits in risk factor detection, treatment and control are well recognized. The ‘rule of halves’ has been applied to hypertension management, which suggests that half of hypertension may be detected, with half of these treated and half controlled,8 and some studies also suggest that hypertension may be less well controlled in obese patients.9, 10, 11, 12
The growing obesity epidemic is a major source of unsustainable health costs and morbidity and mortality because of hypertension, type 2 diabetes mellitus, dyslipidemia, certain cancers and major cardiovascular diseases. The world is experiencing a real noncommunicable disease emergency, which has been precipitated by unhealthy lifestyle characteristics (that is, physical inactivity, smoking, unhealthy diet and excess body mass) and associated poor health metrics (hypertension, dyslipidemia and hyperglycemia).13, 14, 15, 16 The American Heart Association has clustered these seven key health factors into a singular concept, life’s simple 7, with each factor being designated into one of the three categories—poor, intermediate or ideal cardiovascular health.16 We now recognize that improving life’s simple seven characteristics on a population level is the only way the noncommunicable disease crisis can be resolved.17 The United States is currently facing a very real obesity epidemic. The most recent National Health and Nutrition Examination Survey indicates that approximately two-thirds of US adults are presently classified as overweight or obese (BMI⩾25 kg m−2) and one-third as obese (BMI⩾30 kg m−2).18, 19 Although the numbers alone are formidable, they leave unaddressed the medical costs associated with obesity and obesity-related comorbidities, not the least of which is obesity-related hypertension. Given the frequent concurrence of obesity and hypertension, it is no coincidence that, as the rate of obesity continues to rise, so too does the rate of hypertension. It is estimated that at least 75% of the incidence of hypertension is related to obesity.18 It is essential, therefore, to develop treatment strategies for the management of obesity in order to reduce the development of obesity-related hypertension, as well as to effectively manage high blood pressure, in the obese.
Body habitus is a key lifestyle characteristic whose current status and future projections are highly disconcerting.20, 21 Much of the world has evolved into a positive caloric balance culture, and excess body mass is the consequence; paradoxically, nearly 800 million people are malnourished at the very opposite. Globally, the percentage of the population, both children and adults, who are either overweight (that is, BMI 25.0–29.9 kg m−2) or obese (that is, BMI ⩾30 kg m−2) has substantially increased over the past three decades; there is no indication any country has a solution to this issue.22 In 2014, it was estimated that 1.3 billion adults around the world were overweight and 600 million were obese; the worldwide prevalence of obesity doubled from 1980 to 2014. In the United States, the percentage of individuals who are considered obese has, for the first time, surpassed the percentage of individuals classified as overweight.20 It is estimated that obesity was the cause of 18.2% of the deaths between 1986 and 2006 in the United States.23 Globally, it is estimated that 5% of the annual deaths are caused by obesity. In 2010, excess body mass ranked sixth among 67 risk factors that accounted for global disease burden.24 Although lifestyle changes aimed at prevention, especially in childhood, are the ultimate solution to the societal problem of obesity and its complications, the scope of illness caused by obesity demands immediate attention and therapeutic intervention in the obese population, also in light of the important role that obesity has in the pathogenesis of hypertension.
Similar to obesity, hypertension is a key unfavorable health metric that has disastrous health implications if left uncontrolled.21, 25 In 2008, it was estimated that 40% of the global adult population (⩾25 years) had elevated blood pressure with approximately 1 billion cases of uncontrolled hypertension, a 400 million individual increase from 1980. In the United States, 32.6% of adults have hypertension, ≈80 million individuals.21 Although 76.5% of these individuals in the United States with hypertension are being treated for this health metric, only 54.1% are controlled effectively; 17.3% of US adults are not aware they have hypertension.21 In 2011, 65 123 deaths were attributable to in the United States, and there were 377 258 any-mention deaths for hypertension21; globally, hypertension accounts for 9.4 million deaths annually. Currently, hypertension is the leading contributor to global disease burden,26, 27 and the direct and indirect cost of treating hypertension in the United States and worldwide is exponentially growing. Poor lifestyle characteristics and health metrics often cluster together to create complex and difficult-to-treat phenotypes. Excess body mass is such an example, facilitating a cascade of pathophysiological sequelae that create such as a direct obesity–hypertension link, which consequently increases cardiovascular risk.24, 28, 29
Although some argue that obesity could be classified as a disease,30 some significant issues regarding assessment/management of obesity remain to be addressed. The first issue is that obesity is measured by various means, such as BMI, waist circumference, waist-to-hip ratio or by assessing visceral adiposity by imaging techniques. This means that the diagnosis of obesity could be influenced by the index used, also given that it is already well established that there are considerable variations in the waist circumference and visceral adipose tissue at any given BMI value.31, 32 The second issue is that, although World Health Organization proposed lower BMI cutoff points for obesity among Asians,33 cohort studies about the relationships between BMI and mortality do not seem to consistently support the need for lower BMI thresholds for the Asian population.34, 35, 36 Finally, within the Asian population itself, there may be differences in body composition and adipose tissue distribution.
The more critical issues are the heterogeneity of obesity as a phenotype as it has been proposed by some investigators and that there may even be a healthy form of obesity referred to as ‘metabolically healthy obesity’ (obesity that is characterized by a normal metabolic risk profile).37 In addition, the reported protective effect of obesity in patients with cardiovascular disease, commonly referred to as the ‘obesity paradox’, poses an additional challenge to the cardiovascular risk assessment and management of the obese population.38 Finally, the suggested protective role of subcutaneous adipose tissue also support the importance of adipose tissue quality and function rather than just the level of adiposity per se in the regulation of lipid and carbohydrate metabolism and related cardiometabolic risk profile.2, 39, 40, 41
Despite the unique characteristics of adipose tissue as an essential endocrine organ to maintain energy homeostasis, there is no doubt that obesity is harmful, is associated with a plethora of health problems and definitely leads to increased risk of mortality at the population level.42, 43, 44 However, the presence of the obesity paradox in cardiovascular disease has left some clinicians quite perplexed about the ‘identity’ of obesity. On the other hand, although the underlying mechanisms governing these disparate effects of obesity on cardiovascular disease are complex and not completely understood, a variety of factors, such as different anthropometric indices, body fat distribution, body composition, muscle mass and strength and cardiorespiratory fitness, appear to have a critical role in explaining the paradoxical association of obesity with clinical outcomes. Consequently, a comprehensive and exhaustive investigation of complex and disparate effects of obesity on cardiovascular disease should also discuss whether or not metabolically healthy obesity exists and examine factors potentially involved in explaining the obesity paradox, in the aim to fully describe and understand the obesity–hypertension link and discuss strategies to address the potential negative consequences from the perspective of both primordial prevention and treatment for those already impacted by this condition.
Mechanisms and pathophysiology of obesity-related hypertension
The association of obesity and hypertension has been recognized since the beginning of the twentieth century when blood pressure was first measured in populations, and this relationship between body weight and blood pressure was demonstrated prospectively in the Framingham Heart Study in the 1960s.45 The nature of the linkage between blood pressure and body weight remained obscure until the mid-1980s when basic clinical and population-based research significantly clarified many aspects of the relationship between these two common and complex regulatory disturbances. Appreciation of the clinical significance of obesity-related hypertension has grown substantially over this same time period, to the point where obesity is recognized as a major cause of high blood pressure, and the combination of obesity and hypertension is recognized as a preeminent cause of cardiovascular risk.
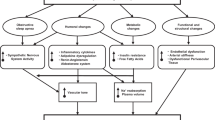
Clues to the basic mechanisms involved in the link between obesity and hypertension first appeared in the 1940s and 1950s with the important observations by Vague.46 Based on observations made in his own obesity practice, Vague noted that the cardiovascular and metabolic complications of obesity were more common in patients with the upper body obesity phenotype, which he called ‘android’, as compared with lower body obesity, which he referred to as ‘gynoid’. These prescient observations attracted little attention until the 1980s when population-based studies using waist-to-hip ratio as a quantifiable surrogate for the upper body phenotype demonstrated significant cardiovascular risk (hypertension, myocardial infarction, dyslipidemia and type 2 diabetes mellitus) in association with a high waist-to-hip ratio.47, 48, 49 Similar lines of research showed that insulin resistance was also associated with the upper body phenotype50, 51, 52 and many subsequent clinical and population-based studies showed an association of insulin levels and/or insulin resistance with hypertension in both obese and non-obese people.53, 54 Thus insulin, hypertension and the android or central obesity phenotype tracked together in population-based and clinical studies. These observations formed the basis for our current understanding of the pathophysiology of obesity-related hypertension.55 There is a clearly established link between obesity and hypertension.28, 29, 56, 57 The accumulation of excess adipose tissue initiates a cascade of events that give rise to an elevated blood pressure; obesity-induced hypertension is a common pathway in both children and adults.29, 58, 59 Pathogenetic factors and pathophysiological mechanisms linking obesity to hypertension (Figure 1) are described and reviewed herein as they provide the basis for a rational therapeutic strategy.
Mechanisms involved in the pathogenesis of obesity-induced hypertension. AgRP, agouti-related peptide; ARC, arcuate nucleus; CRP, C-reactive protein; ET-1, endothelin-1; FFAs, free-fatty acids; ICAM-1, inter-cellular adhesion molecule-1; IL-6, interleukin-6; IL-1β, interleukin-1β; MC3R, melanocortin 3 receptor; MC4R, melanocortin 4 receptor; NO, nitric oxide; NPY, neuropeptide Y; PAI-1, plasminogen activator inhibitor-1; POMC, proopiomelanocortin; RAS, renin–angiotensin system; ROS, reactive oxygen species; SNS, sympathetic nervous system; TNFα, tumor necrosis factor-α; Tx-A2, thromboxane A2; VCAM-1, vascular cell adhesion molecule-1; α-MSH, α-melanocyte-stimulating hormone; Reproduced/modified from Kotsis et al.
Increased sympathetic nervous system activity, adipokines and insulin
There is an increase in sympathetic nervous system activity in patients with obesity; evidence suggests that high caloric loads increase peripheral noradrenaline turnover, and high fat and carbohydrate diets stimulate α1 and β-adrenergic peripheral receptors, which elevates sympathetic nervous system activity.29, 60 Elevated free fatty acid levels, typical of the obesity–hypertension phenotype, increase α-adrenergic vascular sensitivity and subsequently arterial tone. Distribution of body fat also has a role in sympathetic nervous system variability, with central obesity being associated with greater sympathetic nervous system activation compared with subcutaneous obesity.61 Finally, baroreflex sensitivity, which when functioning normally has a sympathoinhibitory effect in elevated blood pressure conditions, is diminished in the obesity–hypertension phenotype, further contributing to enhanced sympathetic nervous system activity.62
The adipocyte as an active endocrine secretory cell, as well as an immune organ, produces many different adipokines to modulate inflammation and various metabolic processes.63, 64 In normal physiological circumstances, adipocytes release anti-inflammatory factors such as adiponectin, transforming growth factor-beta, interleukin-10 and nitric oxide, which promote insulin sensitivity and antiatherogenic effects.63, 64 At the population level, adiponectin could be found in high levels in the blood of lean healthy individuals, whereas its concentration was markedly reduced among individuals with type 2 diabetes, coronary heart disease or with a high-risk form of overweight/obesity.65 As a result, adiponectin has generally been perceived as an antidiabetogenic/antiatherosclerotic adipokine; on the contrary, pathological hypertrophied adipocytes caused by excessive body weight release pro-inflammatory cytokines such as leptin, tumour necrosis factor-alpha, resistin and interleukin-6, contributing to the development of various metabolic diseases.66 These contrasting effects are maintained in balance in a normal state, although increased levels of adiponectin have been linked to higher mortality in older adults or patients with cardiovascular disease, which is termed as the ‘adiponectin paradox’,64 and recent data reported that increase in leptin level had conferred some beneficial effects on coronary endothelial function.67 Adiponectin is secreted by adipose tissue, has a role in regulating energy balance and promotes insulin sensitivity. Increased adipose mass is associated with decreased adiponectin levels and thus contributes to insulin resistance. Glucocorticoids, acting within adipose tissue, appear to promote hypertension through increased renin–angiotensin–aldosterone system activity.29, 59 Leptin is secreted by adipose tissue, with a direct link between leptin secretion and fat mass.29, 59, 68 Elevated circulatory leptin levels observed in obesity are also implicated in the increased risk of hypertension. Leptin crosses the blood–brain barrier, interacting with the arcuate nucleus and, similar to insulin, initiating an appetite suppression and increased energy expenditure signal that is mediated through increased sympathetic nervous system activity.69, 70 Leptin also has been linked to endothelial dysfunction by negatively impacting nitric oxide synthase expression and augmenting sympathetic nervous system activity.29, 62
The relationship of insulin to blood pressure, although controversial at first, has a plausible explanation, and insulin is now generally acknowledged to have a role in the pathophysiology of obesity-related hypertension.55 Impaired glucose tolerance, increased insulin levels and concomitant reductions in insulin sensitivity are commonplace in obese individuals; the clustering of these characteristics defines insulin resistance/metabolic syndrome.29, 56, 59, 60 As insulin stimulates the sympathetic nervous system,71, 72 and as obese patients have increased sympathetic nervous system activity,73, 74, 75 a role for insulin-mediated sympathetic nervous system stimulation seems a likely factor in the pathogenesis of high blood pressure in the setting of central obesity, as supported by studies demonstrating concomitant decreases in blood pressure and sympathetic nervous system activity when insulin is lowered by low-energy diets in obese patients.76 Insulin also increases sympathetic nervous system activity through both hypoglycemia-induced mechanisms and a possible direct effect on the central nervous system. Chronic hyperinsulinemia is also linked to arterial dysfunction, favoring vasoconstriction. Moreover, insulin also has a direct action on the kidney to stimulate sodium reabsorption and increase sodium retention through a direct interaction with renal tubules.77 Thus the obesity-induced hyperinsulinemia state contributes to elevated blood pressure through increased sodium retention and volume overload.
Finally, plasma endocannabinoids released from the adipose tissue, such as anandamide and 2-arachidonoylglycerol, remain a focus of attention in obesity research. Endocannabinoids involved in feeding behavior and energy metabolism, as well as glucose and lipid metabolism, are increased via insulin resistance and inflammation in obese individuals, which may stimulate the overexpression of cannabinoid receptors in a pathophysiological manner contributing to excessive visceral fat accumulation and decreases in adiponectin level.78 It is recognized that endocannabinoids may have a key role in the pathogenesis of obesity-related complications, such as nephropathy, atherosclerosis and cardiac dysfunction through inflammation-mediated reactive oxygen species, and lifestyle interventions leading to weight loss and loss of visceral adipose tissue have been shown to reduce anandamide and 2-arachidonoylglycerol levels in the blood of abdominally obese, dyslipidemic men with features of the metabolic syndrome.79
Alterations in renal function, sodium excretion, pressure natriuresis, salt sensitivity and renin–angiotensin–aldosterone system
Obesity is associated with an increased risk for chronic kidney disease, as well as end-stage renal disease.80 Initially, blood pressure control through diuresis and natriuresis favors a shift toward hypertension in obese individuals; this occurs prior to glomerular injury and loss of renal function.29, 59, 81 During the initial onset of obesity, an increase in renal tubular reabsorption increases sodium retention. Renal vasodilation, which increases glomerular filtration and the filtered amount of both water and electrolytes, occurs in an attempt to compensate for the increase in renal tubular reabsorption. This compensation is incomplete, however, and extracellular volume is expanded with an upward pressure recalibration of the pressure natriuresis. Thus the impact of obesity on the renal system that favors hypertension is consistent with a volume overload model. Obesity predisposes the kidney to reabsorb sodium by neural (sympathetic nervous system), hormonal (aldosterone and insulin) and renovascular (angiotensin II) mechanisms.82 This enhanced sodium avidity shifts the pressure natriuresis curve to the right,83 thereby necessitating higher arterial pressure to excrete the day’s salt intake and maintain sodium balance and volume homeostasis. This is the basis for the documented salt sensitivity of obesity-related hypertension84 and underlines the need for diuretics in the therapeutic regimen.
Obesity is associated with altered/activated renin–angiotensin–aldosterone system function; plasma renin, angiotensinogen, angiotensin II, and aldosterone are all elevated with obesity, and aldosterone levels may be increased out of proportion to the increase in renin activity.85 Several mechanisms have been thought to underlie renin–angiotensin–aldosterone system activation, including sympathetic nervous system stimulation of renin release86 with the generation of angiotensin II; angiotensinogen production in adipose tissue, especially intra-abdominal adipocytes86, 87 with the generation of angiotensin II and aldosterone; and effects of free fatty acids, along with other poorly defined factors, on aldosterone production and release.85 Increased levels of renin–angiotensin–aldosterone system constituents favor vasoconstriction and volume expansion. Although obesity is associated with volume expansion, renin secretion by the kidney persists because of the effect of fat accumulation in and around the renal medulla. Adipose tissue itself is also a source of all components of the renin–angiotensin–aldosterone system;29, 56, 88 angiotensinogen produced by adipose cells is released into the circulatory system, increasing the amount available for conversion along the pressure elevating cascade, and renin–angiotensin–aldosterone system receptors are well established in adipocytes.88 This cascade of events results in elevated renin–angiotensin–aldosterone system activity, which is no longer suppressed by the obesity-associated volume expansion. Excess visceral adipose tissue results in physical compression of the kidneys. Compression of the renal system impacts both the vascular and tubular systems that augment renin–angiotensin–aldosterone system activation and sodium reabsorption.59 The physical stresses that obesity places upon the kidneys initiate a deleterious progression from hyperfiltration to glomerulomegaly (that is, enlargement of the glomeruli) to sclerosis of the glomeruli wall and nephron, ultimately leading to nephron loss, which negatively impacts pressure natriuresis.58 These structural renal changes result in higher sodium retention and higher arterial pressure.
Other potential mechanisms
Other factors that may be implicated in the pathophysiology of obesity-related hypertension include a decrease in natriuretic peptides85, 86 with consequent impairment in salt excretion; a decrease in adiponectin;89 obstructive sleep apnea, which stimulates the sympathetic nervous system;90, 91, 92, 93 low birth weight, which is associated with excessive weight gain in childhood and adolescence, along with increased risk of hypertension and stimulation of the sympathetic nervous system94, 95, 96 in adulthood; and endothelial dysfunction with consequent blunting of physiological vasodilation.97, 98 Particularly about endothelial function and structure, obesity creates a state of insulin resistance and systemic inflammation that promotes endothelial dysfunction and hypertension.29, 59 Insulin resistance decreases nitric oxide synthesis and hyperinsulinemia promotes vasoconstriction through increased endothelin-1 levels. A host of proinflammatory and inflammatory compounds secreted by adipose tissue, including interleukin-1β, interleukin-6, tumor necrosis factor and C-reactive protein, also promote endothelial dysfunction and thus hypertension.29 Obesity is also associated with increased carotid artery thickness, intima media thickness and arterial remodeling and stiffening.99 Chronic hyperglycemia, as well as increased renin–angiotensin–aldosterone system activation and sympathetic nervous system activity, contributes to changes in vascular structure that favor blood pressure increase and development of hypertension.
Epidemiology and prevalence of obesity-related hypertension
In the United States, more than 40% and 25% of the obese and overweight population, respectively, also have hypertension. This, compared with a hypertension prevalence of approximately 15% in normal-weight individuals,4 clearly demonstrates a stepwise increase in hypertension risk with increasing body mass. It is estimated that 78% of the risk for developing essential hypertension in men and 65% of the risk for developing essential hypertension in women is attributed to excess body mass.59 Evaluating the relationship from the reverse perspective (that is, the hypertension population exclusively), >70% of individuals with hypertension are overweight or obese.100 In those with type II diabetes mellitus, the prevalence of the obesity–hypertension phenotype is variable by country and defining blood pressure threshold, ranging from 33% to 93%.101 Collectively, these data indicate a high prevalence for the obesity–hypertension phenotype, warranting a focus on prevention and treatment.
Data from recent US National Health and Nutrition Examination Surveys (NHANES) from 2005 to 2008 indicate that the prevalence of hypertension among adults aged >18 years in the United States was 30.9%, or nearly 1 in 3 adults. In the context of the entire population, >76 million US adults are estimated to have hypertension; at the same time, nearly 70% of American adults are overweight or obese.102 Thus we can expect a significant increase in the prevalence of hypertension in the coming years if trends of increasing weight in the population are not stabilized and reversed. Epidemiological data unequivocally support the link between body weight and blood pressure, thus indicating greater body weight as one of the major risk factors for high blood pressure. Recent data from NHANES indicate that the prevalence of hypertension among obese individuals, with a BMI⩾30 kg m−2, is 42.5% compared with 27.8% for overweight individuals (BMI 25.0–29.9 kg m−2) and 15.3% for those with BMI<25 kg m−2.103 Likewise, higher BMI is also associated with increased risk for development of hypertension over time. Data from the long-standing Framingham Heart Study revealed that, compared with normal-weight adult men and women, the multivariable-adjusted relative risks for development of hypertension in long-term follow-up were 1.48 and 1.70 for overweight men and women and 2.23 and 2.63 for obese men and women, respectively.104 With the current obesity epidemic extending into its third decade, prevalence rates of hypertension, which had been falling in the 1970s and 1980s, are again rising.
Numerous studies have also demonstrated the important role of weight gain in blood pressure elevation and of weight reduction in blood pressure lowering. As a general rule, in Western societies, systolic blood pressure and diastolic blood pressure tend to rise with age beginning at around age 25 in most adults.105, 106 This is a ‘normative’ process of aging, but it may not be inevitable or ‘normal’. In fact, recent data suggest that these ‘age-related’ increases in systolic blood pressure and diastolic blood pressure may be avoided in young adults who maintain stable BMI during long-term follow-up into middle age. In the Coronary Artery Risk Development in Young Adults study, young adults (mean age 25 years at baseline) who maintained a stable BMI (within 2 kg m−2 of baseline) at six examinations during 15 years had no significant changes in systolic or diastolic blood pressure, whereas those who had an increase in their BMI⩾2 kg m−2 had substantial increases in blood pressure.107 For example, women who maintained stable BMI in this study had nonsignificant declines in systolic blood pressure, whereas women whose BMI increased had statistically significant average increases in systolic blood pressure of 9.8–12.5 mm Hg. Of note, this weight gain was more important than the baseline weight, as the same patterns were observed for those who were at normal weight or overweight at baseline. Hence, age-related changes in blood pressure may not be inevitable and may be caused more by age-related weight gain than aging per se. These data have important implications for health care and public health, as weight maintenance may be a strategy that is easier to achieve than substantial weight loss for preventing or controlling hypertension.107 The influence of weight gain on blood pressure and the benefits of maintaining stable weight or losing weight extend down even to young children. One large birth cohort study of children examined BMI at ages 5 and 14 years and the association with systolic and diastolic blood pressure at age 14 years. Children who were overweight at age 5 years but had normal BMI at age 14 years had similar mean systolic and diastolic blood pressure to those who had a normal BMI at both time points. Conversely, children who were overweight at both ages or who had a normal BMI at age 5 years and were overweight at age 14 years had higher systolic and diastolic blood pressure at age 14 years than those who had a normal BMI at both ages, even after adjustment for potential confounders.108
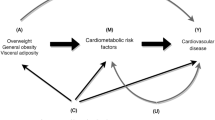
Joint effects of obesity and hypertension on cardiovascular risk
Hypertension is a complex phenotype that arises from numerous genetic, environmental (including air pollution26), behavioral and even social origins, and obesity is one of the most prevalent risk factors for its development. Regardless of its etiology, however, hypertension is a highly prevalent and highly significant risk factor for the development of all manifestations of cardiovascular disease, including coronary heart disease, stroke, heart failure, aortic and peripheral arterial disease, and valvular heart disease. The association of hypertension with cardiovascular risk in the short and long term is unequivocally established. The association of obesity with short-term cardiovascular disease event rates (for example, in the next 10 years) is more difficult to establish, largely because the major effects of obesity appear to act through more proximal risk factors, such as diabetes, dyslipidemia and hypertension. However, longer-term studies of obesity and cardiovascular disease do indicate risk for cardiovascular disease associated with obesity independent of these other risk factors. In addition, several lines of evidence data suggest that obesity and hypertension may have additive effects in increasing risk for cardiovascular disease over long-term follow-up.109 Data from the long-standing Chicago Heart Association Detection Project in Industry,110 which enrolled >38 000 individuals from 1967 to 1973, serve as an example to highlight these joint risks. As reported, 32-year cardiovascular disease death rates were higher (and increased in a stepwise manner) for patients with higher BMI at baseline and no hypertension. For those with hypertension at baseline, cardiovascular disease death rates were substantially higher overall and increased in a stepwise manner for patients with higher baseline BMI levels. A similar pattern of results was observed for individual outcomes of coronary heart disease death and stroke death rates, as well as for hospitalizations for coronary heart disease, stroke and heart failure during follow-up using Medicare data.
An additional important factor in the cardiovascular risk associated with obesity and hypertension is the role played by obesity in the development of type 2 diabetes. Obesity, and particularly central adiposity, is the dominant risk factor for the development of type 2 diabetes, which routinely clusters with hypertension because of common underlying pathophysiology. Diabetes exerts a substantial independent and amplifying effect on the cardiovascular risks associated with both obesity and hypertension.111, 112 Efforts aimed at diminishing the incidence and impact of diabetes, therefore including both lifestyle changes and the appropriate use of antihypertensive and antiobesity therapies, are an essential part of the overall therapeutic plan.
Metabolically healthy obesity and obesity paradox
Although being overweight and obese are well-established risk factors for cardiovascular disease, there are substantial individual differences observed in the cardiometabolic risk profile of subjects within the same BMI category. Based on favorable metabolic features observed in some obese patients such as high levels of insulin sensitivity and high-density lipoprotein-cholesterol as well as low levels of fasting triglycerides and fasting glucose, a unique obesity phenotype known as ‘metabolically healthy obesity’ was introduced.113, 114 Early studies suggested that metabolically healthy obesity was not associated with an increased risk of cardiovascular mortality compared with normal-weight individuals.115 Such early data have been interpreted by some as providing evidence that these apparently metabolically healthy obese people would not need to receive preventive therapies, because they would appear unlikely to experience long-term morbidity on the basis of their seemingly normal cardiometabolic risk profile.116 However, considerable controversy exists about the criteria and levels to be used to define metabolically healthy obesity and whether obesity can be healthy. Recent studies with long-term follow-up information have refuted the existence of metabolically healthy obesity by demonstrating that these individuals are nevertheless associated with an increased risk of cardiovascular disease.117 In addition, a series of cross-sectional studies have shown that metabolically healthy obesity was associated with subclinical target organ changes, including increased carotid intima media thickness and coronary artery calcium scores, subtle impairment of left ventricular structure and function and impaired vasoreactivity.118, 119, 120, 121 Several potential explanations for these conflicting results have been proposed in recent years. First of all, there is no standard definition for metabolically healthy obesity. Although Hinnouho et al.122 suggested that metabolically healthy obese individuals were at increased risk of mortality, irrespective of various definitions used, and these different definitions still make the comparison of findings among studies, such as prevalence rates and the long-term health effects, difficult.123, 124 Second, the issue of a difference in the follow-up duration between studies is another crucial factor affecting the study results. Most studies supporting the existence of metabolically healthy obese phenotype have had a relatively short follow-up period of <10 years, whereas data by Arnlöv et al.117 have shown that there was an increased risk for cardiovascular disease events in metabolically healthy obese subjects when a follow-up period beyond 10 years and up to 30 years was used. Similarly, Lee et al.125 demonstrated that the metabolically healthy obese group showed a similar risk of incident hypertension during the first 4-year follow-up compared with metabolically healthy normal-weight controls. However, an increased risk of developing hypertension began to be observed only after 6-year follow-up. In addition, the authors refuted the concept of ‘benign obesity’ by demonstrating that a higher proportion of metabolically healthy obese group deteriorated metabolically over time.126 Indeed, they observed a progressive conversion of metabolically healthy obese subjects from being healthy to becoming unhealthy over the follow-up period.125 Thus these results emphasize the long-term cardiovascular hazards of being characterized by an apparently benign obesity phenotype and the necessity to follow these patients over the long term. In other words, although younger individuals may have metabolically healthy obesity, they may nevertheless develop insulin resistance, diabetes, dyslipidemia and arterial hypertension called metabolic syndrome with increasing age.
Recently, two meta-analyses concluded that the metabolically healthy obese phenotype should no longer be considered as a benign condition. One analysis, including 8 studies in 61 386 people with a follow-up period of >10 years, reported that metabolically healthy obesity was associated with an increased risk for all-cause mortality and cardiovascular disease events.126 Another study also demonstrated similar findings: in a meta-analysis of 14 studies involving 299 059 individuals, a 100% increased risk for cardiovascular disease events was observed in the metabolically healthy obese group.127 The risk of clinical outcomes was much worse when only considering studies with >15-year follow-up. Accordingly, by labeling a subset of obese people as metabolically healthy, a strategy of only offering treatment to obese patients with overt metabolic derangements would seem to provide a short-sighted view to the current obesity epidemic.128 In addition, according to a recent study by Ortega et al.,129 when physical activity or fitness was considered as one of the confounders, the adverse effect of metabolically healthy obese phenotype on all-cause and cardiovascular disease mortality was significantly attenuated or even not observed. In other words, as a high level of cardiorespiratory fitness was associated with a low incidence of metabolic syndrome and attenuated the magnitude of the relationship between the metabolic syndrome and mortality regardless of weight status,130 these results suggest that the metabolically healthy obese phenotype is clearly associated with a higher level of cardiorespiratory fitness.131 These findings could provide useful information to physicians in assessing the risk stratification of obese individuals. Thus the prevalence of the metabolically healthy obesity phenotype is probably much lower than expected and levels of physical activity/cardiorespiratory fitness are probably some of the key factors, in addition to body composition and level of visceral adiposity/ectopic fat, to explain this phenomenon. Our suggestion would be to define metabolically healthy not only on the basis of absence of any criteria of the metabolic syndrome but rather as having all usual risk factors being at optimal levels; otherwise, any slight increase in any of the risk factors increases the risk of cardiovascular disease.
Clinical research reveals an obesity paradox, where overweight/obese patients with hypertension, heart failure, coronary heart disease and peripheral arterial disease have perhaps even a more favorable short- and long-term prognosis. Notably, a large cohort study of 22 576 treated hypertensive patients with known coronary heart disease demonstrated that all-cause mortality was 30% lower in overweight/obese compared with normal-weight patients.132 Moreover, the landmark Systolic Hypertension in Elderly Program showed decreased stroke and total mortality among overweight compared with lean patients.133 Therefore, although obesity is a powerful risk factor for hypertension and left ventricular hypertrophy, obese hypertensive patients may paradoxically have a better prognosis compared with lean patients.134 Similarly, with heart failure, obese patients may have a better event-free survival.135 There is strong and powerful evidence showing that an elevated BMI predisposes individuals to most cardiovascular disease and has been associated with a greater risk of mortality. In a 10-year follow-up study of 527 265 US men and women in the National Institutes of Health-American Association of Retired Persons cohort between the ages of 50 and 71 years,42 a J-shaped relationship between BMI and the risk of death was observed, showing that underweight and excess body weight, including overweight, were associated with higher rates of death than normal weight. On the other hand, a recent meta-analysis from prospective studies of general populations reported a statistically significant 6% lower all-cause mortality in overweight persons compared with normal-weight subjects. Although the mortality risk was increased in overall obesity including all classes (class I, II and III combined) and class II (BMI 35–40 kg m−2) and class III obesity (BMI⩾40 kg m−2), class I obesity (BMI 30–35 kg m−2) was not associated with higher mortality compared with normal-weight individuals;136 however, considering the fact that the mortality rate for a BMI between 19 and 20 kg m−2 is higher than a BMI between 24 and 25 kg m−2 where the lowest mortality is observed, the use of a wide range of BMI between 18.5 and 25 kg m−2 as the presumably ‘normal weight’ reference group could explain the protective effect conferred by overweight and class I obesity in the general population. Recent data from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial confirmed that the protective effect of obesity on all-cause mortality was not observed after excluding individuals with BMI<22 kg m−2 who are clearly at increased risk compared with subjects in a normal but higher BMI range such as 22–25 kg m−2.137 Nevertheless, there is accumulating evidence that obesity in patients with established cardiovascular disease, including coronary heart disease, heart failure, hypertension and atrial fibrillation, has a protective role against both all-cause and cardiovascular mortality, findings which contributed to feed the ‘obesity paradox’ hypothesis in the field of cardiology.138 However, because this obesity paradox is being reported in a variety of chronic diseases including end-stage renal disease, chronic obstructive pulmonary disease and type 2 diabetes mellitus, it does not appear to be specific for only cardiovascular disease.
A variety of mechanisms have been proposed to explain the obesity paradox in patients with cardiovascular disease.138 Among potential causes for the obesity paradox, spontaneous weight loss, which is observed after the development of heart failure, is known to cause a bias associated with the timing of weight measurement. However, because a recent study from the Atherosclerosis Risk In Communities has suggested that the protective effect of obesity paradox is driven by preexisting obesity,139 cardiac cachexia due to advanced heart failure does not appear to fully explain the obesity paradox in patients with established heart failure, although this phenomenon certainly contributes to the high mortality risk of very lean heart failure patients. Given that the obesity paradox is usually observed in the elderly with cardiovascular disease, age-related changes of body fat distribution and also sarcopenia may be clues to the obesity paradox.2 Accordingly, the concept of being fat and fit has recently come into the spotlight as a potential reason for the obesity paradox.138
The metabolic syndrome
Obesity-related hypertension frequently occurs in association with other cardiovascular risk factors, forming a constellation referred to as the metabolic syndrome.140 Although the concept of a metabolic syndrome has achieved widespread acceptance over the past two decades, no consensus has developed over the precise definition of the syndrome nor over the criteria required to establish the diagnosis. No less than five sets of diagnostic criteria have been proposed by different international and national panels, including the International Diabetes Federation, the World Health Organization and the US National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III), among others.141 The differences in criteria, although small and overlapping, point to the imprecision in defining the metabolic syndrome and to the differences in the perceived importance of the various manifestations. Using the NCEP-ATP III criteria and the NHANES III survey, it has been estimated that about 30% of the US population has the metabolic syndrome.142 The prevalence increases with age so that by 60 years, >40% of people meet criteria for the diagnosis.
The four fundamental components of the metabolic syndrome are central obesity, insulin resistance, hypertension and a characteristic dyslipidemia (high triglycerides and low high-density lipoprotein cholesterol). Reaven141 has identified the importance of insulin resistance as a critical component of the syndrome, which he originally designated ‘syndrome x’ and which was also called the ‘insulin resistance syndrome’, although these earlier designations have given way to the term metabolic syndrome. Central or android obesity is the usual although not the exclusive cause of the insulin resistance. Insulin resistance and the consequent hyperinsulinemia drive the hypertension (as described above) and the dyslipidemia (stimulation of hepatic very low-density lipoprotein production). In addition to the four principal components, a variety of other abnormalities have been associated with the metabolic syndrome, including type 2 diabetes mellitus, impaired glucose tolerance, renal functional impairment and microalbuminuria, hyperuricemia, prothrombotic coagulation diatheses, small dense low-density lipoprotein cholesterol and markers of inflammation.143 All of these abnormalities have been associated with increased cardiovascular risk.
Although abdominal obesity and insulin resistance are the major threads that connect the various features of the metabolic syndrome, two other factors of potential importance should be mentioned: dietary fructose and disordered sleep. The consumption of high fructose corn syrup has increased dramatically in the past three decades, paralleling the increase in obesity and hypertension. Sweetened beverages, such as non-diet soda, account for 70% of the intake of high fructose corn syrup,144, 145 and recent evidence suggests a link between sweetened sodas, hyperuricemia and the manifestations of the metabolic syndrome.146, 147
Another recently described factor that may contribute to the development of the metabolic syndrome is shortened or interrupted sleep. Obstructive sleep apnea, a well-recognized complication of obesity, is associated with increased sympathetic nervous system activity, which persists during daytime wakefulness.90, 92 The sympathetic nervous system overactivity is associated with hypertension. Both the sympathetic nervous system stimulation and the hypertension are reversed with effective treatment of the sleep apnea. Sleep debt, a consequence of shortened or disordered sleep, or night shift work, is also associated with obesity, insulin resistance and hypertension, raising the possibility that disturbances of normal sleep patterns may have a role in the pathogenesis of the metabolic syndrome.93, 148, 149, 150 Insufficient sleep may also antagonize weight loss in response to caloric restriction.151
Considerable debate has centered on whether the metabolic syndrome is in fact a discrete entity. Although the abnormalities characteristic of the metabolic syndrome are relatively common in the population at large, there is no doubt that these traits occur together much more commonly than predicted by chance alone. This, however, may reflect the central role of insulin resistance and hyperinsulinemia in the pathogenesis of the different manifestations, rather than a direct linkage of the associated traits. The argument has been advanced, with some merit, that the concept of a metabolic syndrome may alert clinicians to look for associated manifestations when obesity and hypertension coexist and to recognize the cardiovascular risk associated with this constellation.
Prevention and treatment options for the management of obesity and hypertension
The obesity–hypertension phenotype works synergistically to exponentially increase cardiovascular disease risk, chronic renal disease and associated adverse events.4, 21, 81, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161 Given the incidence and prevalence, poor clinical outcome and quality of life as well as negative financial implications of the obesity–hypertension phenotype, aggressive prevention and treatment strategies are imperative.
Lifestyle changes in the management of obesity-related hypertension
The importance of lifestyle management in the treatment of patients with obesity-related hypertension cannot be misunderstood. Adoption of a healthy lifestyle facilitates weight loss, increases responsiveness to antihypertensive medications and produces independent beneficial effects on cardiovascular risk factors.
Promoting the maintenance of a life-long normal body weight is the optimal approach to preventing the deleterious consequences of the obesity–hypertension phenotype. If obesity were removed from the equation, the risk of developing hypertension would be minimized for a significant proportion of the population. The prevention of obesity from occurring requires a multipronged approach by numerous stakeholders working together toward a common goal. The focus is to encourage adoption of a healthy lifestyle from the individual to population level across the lifespan. The core tenets of a healthy lifestyle as it relates to maintenance of a normal body weight include sufficient levels of physical activity and consuming a nutritious, calorically balanced diet. The American Heart Association has defined ideal physical activity and dietary characteristics that all individuals should strive toward.25 Recently, the American Heart Association, European Society of Cardiology, European Association for Cardiovascular Prevention and Rehabilitation and American College of Preventive Medicine published a policy statement that proposed a nonhierarchical connectivity model for key stakeholders who must collaboratively work together to address the noncommunicable disease crisis through healthy lifestyle interventions.17
Professional organizations, educational systems, government on all levels, health-care organizations, the insurance industry, nonprofit and community organizations, media outlets and mobile health and technology companies and employers were all identified as healthy lifestyle stakeholders in this policy statement. With respect to promoting maintenance of a healthy body weight across the lifespan, numerous collaborative strategies can be implemented. Examples include: creation of walker-/biker-friendly public spaces, robust K-12 physical and health education programs as well as healthy food choices in schools, well-designed worksite health and wellness programming, health insurance incentives for maintenance of a healthy lifestyle, and broad adoption of healthy lifestyle assessments and interventions in health-care systems. The aforementioned policy statement encourages creativity in developing healthy lifestyle initiatives, allowing stakeholders to capitalize on resources and infrastructure at the local level. Moving forward, prevention of unhealthy lifestyle characteristics, including obesity, from ever developing must become a primary focus.
The childhood origins of obesity-related hypertension are well illustrated in a study of 260 000 overweight and obese children in Germany and Switzerland, in which 35% had hypertension with increased ventricular mass or arterial stiffness.162 Studies of the Bogalusa childhood cohort who were prehypertensive or mildly hypertensive in adulthood showed that when they were tracked back to as young as 4–8 years, they had higher blood pressures and were heavier and more insulin resistant than their normotensive counterparts.163 This is in accord with prospective studies showing tracking of adiposity, obesity and blood pressures from childhood into adult life. In a recent analysis of four cohort studies followed for a mean of 23 years, overweight or obese children who remained obese as adults had substantially increased risk of hypertension, diabetes, dyslipidemia and carotid atherosclerosis.164 Importantly, patterns of food consumption and physical activity also track from childhood to adulthood.165 Stemming from the work by Barker,94 there is substantial evidence for effects of intrauterine growth and early postnatal weight gain on adiposity and high blood pressure.166 These effects are not restricted to low birth weight infants, as shown by the clustering of adiposity and blood pressure along with impaired glucose tolerance and dyslipidemia in 8- and 14-year olds born from the lowest and highest birth weight quintiles.167, 168 Excessive postnatal weight gain in early childhood dominated over effects of birth weight,168 especially in children of mothers who smoked in pregnancy or did not breastfeed.168 In the same Australian cohort, the trajectories for adolescent obesity were well established by the age of 5 years.169 For all of these reasons, a consensus is developing that it may be necessary to tackle lifestyle-induced obesity-related hypertension at its source: in infancy and early childhood and in the parents.
There are an increasing number of randomized controlled trials attempting to modify and/or prevent childhood obesity at a population level rather than in clinic settings. Most of these have been school based. Few have also examined the effects of the programs on blood pressure. An extensive Cochrane review of lifestyle interventions to prevent obesity in childhood included 55 studies. A meta-analysis of 37 of these involved 27 946 children, of whom the majority were aged 6–12 years,170 concluding that the ‘programs overall were effective at reducing adiposity, although not all individual interventions were effective, and there was a high level of observed heterogeneity and possible bias’. Moreover, the effect was relatively small with children in the intervention group ‘showing a small standardized mean difference in adiposity of −0.15 kg m−2’. Given the unexplained heterogeneity and the likelihood of small study bias, however, these findings must be interpreted cautiously. The authors were unable to distinguish which of the program components contributed to the beneficial effects and suggested that ‘childhood obesity prevention research must now move towards identifying how effective intervention components can be embedded within health, education and care systems and achieve long term sustainable impacts’. None of the above studies reported effects on blood pressure or cardiovascular risk phenotypes other than obesity. However, two large randomized controlled trials of effects of home- and school-based nutrition and physical activity programs on cardiovascular risk factors have been provided from Australia. The first 1147 10–12-year olds from 30 schools found improved blood pressure, a reduction in fatness and improved physical fitness.171 Decline in systolic blood pressure was significantly greater with a fitness intervention for the boys and with a home nutrition intervention for the girls. The greatest improvements overall were with the combined fitness and home nutrition program. The second study used cluster analysis to identify 29% of 800 11-year-old children at increased cardiovascular/metabolic risk. The children in both high- and low-risk clusters were then randomized to two semesters of family nutrition and school-based physical activity programs.172 High-risk children responded better in terms of fatness, fitness, nutrition and blood cholesterol than did low-risk children. Boys responded better than the girls during the program but effects were more sustained after a further 6 months in girls. The lifestyle factors contributing to the rising epidemic of obesity, and hence obesity-related hypertension, are embedded in changes in society worldwide: increased sedentariness from the car, television and computers; parental protectiveness in seemingly hostile urban environments; and increased consumption of calorie-rich foods in the form of soft drinks, fast foods and sugar-enriched low-fat dairy products. Clustering of unhealthy behaviors in obese children, such as poor nutritional habits, high salt consumption, low levels of physical activity and smoking and alcohol consumption (by adolescence)173 dictate the need for multifaceted national-, school- and family-based programs to tackle global cardiovascular risk at an early stage. Many such programs are underway worldwide, and are addressing issues relating to infancy, childhood, parents and the community, and through international networks.174, 175, 176, 177, 178
Lifestyle changes tend not to occur in isolation. Those who are obese tend to show clustering of behaviors predisposing to higher blood pressure including not only disturbed energy balance but less healthy diets with higher salt intake, less fruit and vegetable intake, less low-fat dairy products and increased saturated fat intake,173, 179 sedentary behaviors and in many communities high alcohol consumption. These pro-hypertensive behaviors add to the effects of obesity per se. In industrialized communities, low socioeconomic status is a further factor predisposing to obesity,180 while in developing nations, rising urbanization and westernization with fast-food patterns and decreased physical activity create obesogenic environments.181 Long-term weight gain is insidious, arising from the cumulative effect of excess intake, which may be as little as 50–100 kcal day−1.182 In a longitudinal analysis,105 weight gain in US adults was positively associated with small changes that included increased consumption of sugars, starches, refined grains and processed foods, as well as increased alcohol intake, time spent watching television and decreased physical activity; weight change related inversely to consumption of fruit and vegetables, whole grains, nuts and yogurt. These authors suggest that the small daily changes associated with weight gain could be prevented by small changes in lifestyle adhered to in the long term. Long-term behavior change, however, will need recognition of effective strategies from population studies and clinical trials as well as the cooperation of governments and industry.183 Effects of low socioeconomic status and ethnic differences, with greater predisposition to obesity and hypertension in blacks and obesity in Hispanics, will need particular attention.180, 184
Although we must plan for the future and initiate primordial prevention strategies for life-long maintenance of a normal body weight, the obesity crisis that currently is upon us must also be addressed.185 Weight loss is a key goal for treating patients presenting with the obesity–hypertension phenotype. Additionally, increasing levels of cardiorespiratory fitness has major implications not only for the prevention of hypertension but also for improving overall prognosis in most groups with cardiovascular diseases.186, 187, 188 The literature convincingly demonstrates that individuals with an excess body mass experience significant weight loss through participation in healthy lifestyle interventions that ideally includes a structured exercise program, nutritional counseling and promotion of increased physical activity throughout the day.189, 190, 191, 192, 193, 194, 195 Ensuring these programs are prescribed in a way that creates an appropriate, daily, negative caloric balance (that is, 500–1000 kcal deficit per day) is essential for weight loss and its maintenance.195, 196 With respect to the structured exercise program, combining aerobic and resistance training is considered advantageous with respect to facilitating weight loss while preserving lean mass.192, 195 Behavioral counseling is also considered an important component of healthy lifestyle interventions directed toward promoting weight loss,197, 198, 199 ensuring individuals are properly motivated to lose weight and maintain improvements in body habitus over the long term. Research has shown that individuals who lose weight through exercise and nutritional interventions demonstrate significant reductions in blood pressure, as well as improvements in the pathophysiological cascade associated with the obesity–hypertension phenotype, including improved arterial structure and function.56, 60, 200, 201, 202 In individuals in the early stages of hypertension, exercise training in and of itself, after controlling for baseline body weight and weight loss following an intervention, reduced the risk of developing left ventricular hypertrophy.186, 202, 203 Exercise training also significantly reduces left ventricular mass in those with hypertension where cardiac structural abnormalities have already developed.202, 203 Also, increased physical activity and exercise is particularly important for long-term weight maintenance.204
Weight loss and choice of weight-reducing diet
Systematic reviews consistently report a decrease in systolic blood pressure of about 1 mm Hg per kg of weight loss with follow-up of 2–3 years.205, 206, 207, 208 There is attenuation in the longer-term, with a decrease of about 6 mm Hg in systolic blood pressure per 10 kg of weight loss.206 Intervention programs appropriate for obesity–hypertension combine diet, physical activity and behavioral modification and aim to achieve long-term change in health-related behaviors. In the short term, many variations on reduced-energy diets can achieve weight loss. Diets include very low calorie, balanced deficit (reduction in protein, fat and carbohydrates), changes in a specific nutrient (low fat, low carbohydrate, low glycemic index, high protein) and those popularized through publications or commercial weight-reduction plans.209, 210 Meta-analyses and systematic reviews that compare these various dietary approaches do not favor a specific diet for weight reduction.211, 212
In the management of obesity-related hypertension, a palatable diet rich in components that may lower blood pressure and low in salt is supported by clinical trials.213 Such information has been incorporated in the Dietary Approaches to Stop Hypertension (DASH) diet214 for management of blood pressure, endorsed by the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. This approach focuses on a ‘prudent diet’ rather than the effects of specific nutrients. Appropriate diets for the management of obesity-related hypertension are rich in potassium, calcium and magnesium and fiber and low in salt and saturated fat. In terms of foods, these diets promote consumption of vegetables, fruits, low-fat dairy products, whole grains, nuts, poultry and fish and discourage salt, red meats, sweet foods and sugary drinks. Mediterranean215 and lactoovovegetarian216 diets are also associated with benefits in relation to cardiovascular risk, weight control and blood pressure, but vegetarian diets are not widely acceptable. In US adults, long-term weight gain was related to foods that are discouraged in a prudent diet, while foods promoted in a DASH-type diet were associated with better weight control.179 It is important then to consider diets for weight management in obesity–hypertension in a wider context than energy restriction to ensure an adequate content of foods that may ameliorate blood pressure.213 Several trials have shown early but not sustained benefits of combining DASH-type diets with other modification of lifestyle.217, 218, 219, 220, 221 One study222 found significant differences between white, African American, Chinese and Hispanic groups in conforming to a DASH diet assessed in the Multi-Ethnic Study of Atherosclerosis, indicating that responses may improve if ethnicity is taken into account in delivery of lifestyle programs that include DASH guidelines. The Arthritis, Diet, and Activity Promotion Trial223 included a 3-year follow-up evaluation of a behaviorally based, multifactorial lifestyle program compared with usual care in overweight or obese individuals being treated with not more than two antihypertensive drugs. The 4-month intervention promoted weight loss using a low-sodium DASH-style diet224 with the inclusion of at least four fish meals weekly, moderate-intensity physical activity with increased incidental activity, not more than two standard alcoholic drinks daily and quitting smoking. In the intervention group, weight loss and reduction in waist girth were significantly greater with the intervention after 4 months and 1 year, but decrease in blood pressure was greater only at 4 months. Improvements in diet, notably in fat, sodium, fish and vegetables, persisted to 1 year in the intervention group. Two years later, physical activity was greater in the intervention group and some dietary improvements were maintained but with no significant between-group difference in weight change or blood pressure.
Salt sensitivity is commonly associated with obesity.225 Salt restriction decreases the risk of hypertension with or without weight loss as well as reducing the incidence of cardiovascular events.226 In the Hypertension Prevention Trial,227 participants with diastolic blood pressure 78–89 mm Hg were followed for 3 years after being randomized to one of the five groups: control, decreased energy intake, decreased sodium intake, decreased sodium and energy intake, or decreased sodium and increased potassium intake. Blood pressure decreased in all the groups, with the greatest decrease in patients assigned to reduced energy only. The groups with reduced sodium intake had a significantly lower rate of hypertension. In the Trials of Hypertension Prevention phase I study,228 participants with high normal blood pressure were randomized to one of the four groups for 18 months: control, weight loss, sodium restriction, or stress management. In the weight reduction group, weight decreased by 3.9 kg and blood pressure by 2.9/2.3 mm Hg. Sodium restriction resulted in a decrease in blood pressure of 1.7/2.9 mm Hg. Seven years later, the odds ratio for hypertension among 181 participants was lower by 77% with weight loss and 35% with sodium restriction. Phase II of Trials of Hypertension Prevention examined the effects of weight loss, sodium restriction or both on blood pressure and the incidence of hypertension.229, 230 At 6, 18 and 36 months, weight loss favored the weight reduction intervention over usual care, although weight loss was attenuated over time, and change in blood pressure showed a similar pattern. In the sodium reduction group, a decrease in blood pressure was greater at each time point and also became attenuated with time, from 5.1/4.4 mm Hg at 6 months to 0.7/3.0 mm Hg at 3 years. The Trial of Nonpharmacologic Interventions in the Elderly study231 investigated weight loss and salt restriction and the need for antihypertensive drugs in treated hypertensive patients during follow-up to a median of 29 months. Weight reduction, sodium restriction and the combination of both were compared with usual care in obese participants. The relative hazard ratio was 0.60 for reduced sodium alone, 0.64 for weight loss alone and 0.47 for the combined intervention. The within-groups rate of adverse events was similar.
Physical activity
Aerobic exercise can reduce weight and blood pressure, but when exercise is the only intervention, weight losses are small, with an estimated change of 1.6 kg in moderate-intensity programs continued for 6–12 months.186, 217, 232, 233 In a meta-analysis that included assessment of ambulatory blood pressure,234 it was reported that in studies lasting 4–52 weeks with physical activity as the only intervention, aerobic exercise reduced blood pressure by 3/2.4 mm Hg. The change affected daytime (3.3/3.5 mm Hg) but not nighttime (0.6/1.0 mm Hg) blood pressure. The effect on blood pressure was independent of the estimated weight loss of 1.2 kg. However, when aerobic exercises combined with calorie restriction for weight control, the effects on ambulatory blood pressure can be substantial.235 A few studies234 also examined the effects of resistance training on blood pressure. The estimated decrease in blood pressure (3.2/3.5 mm Hg) was similar to the effects of aerobic exercise, although not statistically significant for systolic blood pressure and without statistically significant weight change. A more recent meta-analysis236 found that resistance training of at least 4 weeks resulted in an estimated decrease of 3.9/3.9 mm Hg in normotensive or prehypertensive individuals, but a decrease of 4.1/1.5 mm Hg in hypertensive patients was not statistically significant. The place of resistance training in programs for management of hypertension is not established. Comparison of high- and low-intensity exercise programs with 3.5–12-month follow-up favored the higher-intensity programs with a difference in weight loss of about 1.5 kg.237 Higher levels of activity may be difficult to maintain in the long term, although the maintenance of 10% weight loss for 2 years in women whose activity increased by 275 min per week from baseline values has been reported.238, 239 Physical activity encourages maintenance of weight loss and offers additional benefits in improving cardiovascular risk factors.239 A systematic review of longitudinal studies of sedentary behaviors in adults found insufficient evidence for an association between sedentary behaviors and measures of adiposity or cardiovascular risk factors, including self-reported hypertension, but there was moderate evidence for an association with type 2 diabetes and strong evidence for an association with all-cause and cardiovascular disease mortality.240 However, longitudinal analysis of data from US adults179 showed that an increase in time spent in watching television predicted weight gain, possibly mediated by lack of physical activity, adverse food choices and eating snacks while watching television. Although there is some variation in the relationships identified, evidence points to the need to incorporate measures to modify sedentary behavior in lifestyle intervention programs.203
Alcohol
A very recent study demonstrated that alcohol abuse increases the risk of incident atrial fibrillation, myocardial infarction and congestive heart failure, exhibiting magnitudes of risk similar to other well-established risk factors, suggesting that alcohol in excess should not be considered cardioprotective but rather cardiotoxic.241 The pressor effect of alcohol has also been established in clinical trials, with an estimated increase in systolic blood pressure of 1 mm Hg per 10 g of alcohol.242 Alcohol provides 29 kJ g−1, and although weight gain from excess intake might be expected, meta-analysis has not shown a consistent relationship between alcohol and weight gain.243 In US adults, however, increased alcohol intake was associated with greater long-term weight gain.179 Moderation of heavier daily alcohol intake to no more than one standard drink in women and two standard drinks in men appears prudent,213 with potential benefits for both weight gain and blood pressure. In a factorial trial of independent and combined effects of alcohol moderation and weight reduction in overweight and obese hypertensive drinkers, effects on blood pressure were additive over a 3-month period, with the combined modalities achieving a 14/9 mm Hg blood pressure reduction compared with controls who maintained usual weight and drinking habits.244
Smoking and behavioral modification techniques
Although smokers tend to have lower body weight, they may gain weight because of clustering of adverse health behaviors.245 Smoking increases blood pressure acutely, with an associated rise in arterial stiffness that lasts longer in hypertensive men.246 There is an important window of opportunity for lifestyle programs to prevent the weight gain (and blood pressure rise) often seen with smoking cessation.179
Behavioral modification techniques are considered an essential part of programs to achieve and maintain weight loss.247 Social and professional support, goal-setting, self-monitoring, stimulus control, changing the environment and problem solving, daily self-weighing and prevention of relapse are strategies that have had some success in improving adherence to weight loss programs.247, 248, 249 In the Weight Loss Maintenance Randomized Controlled Trial,250 improvement in maintenance at 30 months was associated with monthly personal contact with the program staff and regular use of an internet-based intervention.251 Contact by telephone, including text messaging, mail or email, have been used to maintain contact with staff,248 but rapid changes in technology with availability of, for example, social networking and applications for mobile phones offer new opportunities. At this time, there is no consensus about the most effective behavioral strategies for lifestyle modification, particularly in the long term. Trials that are in progress may clarify the best options for encouraging the maintenance of lifestyle change that is so critical to weight control.
Bariatric surgery
Significant weight loss is achieved in individuals who undergo bariatric surgery, and it should therefore be considered in those who are eligible.252, 253 Bariatric surgery is also associated with a significant reduction in blood pressure and improvements in the pathophysiological alterations (for example, sympathetic nervous system, renal system and systemic inflammation) precipitated by obesity.56, 252, 254, 255 In patients undergoing bariatric surgery, healthy lifestyle interventions (that is, exercise training and nutritional counseling) are vital components of the overall care plan to further promote weight loss and maintain a healthy body weight; improve functional capacity and quality of life; and further improve abnormalities in the sympathetic nervous system, renal, hemodynamic, vascular and systemic inflammatory profile associated with the obesity–hypertension phenotype.56, 60, 200, 254, 256, 257, 258, 259 Behavioral counseling should also be integrated into the healthy lifestyle intervention plan for patients undergoing bariatric surgery. Arena and Lavie260 recently proposed broadly embedding healthy lifestyle teams (for example, exercise scientist, registered dietician, behavioral counselor and so on) into the clinical setting to deliver individually tailored healthy lifestyle medicine to those requiring these services. Integrating a healthy lifestyle team into clinical practices caring for patients undergoing bariatric surgery, both preoperatively and postoperatively, is certainly warranted given the importance of healthy lifestyle medicine for this population.
Pharmacological interventions and comorbid disease
Healthy lifestyle interventions and bariatric surgery both can result in a significant reduction in blood pressure in patients with the obesity–hypertension phenotype; significant weight loss is central to achieving blood pressure reductions. Where healthy lifestyle interventions are unsuccessful in producing weight loss and an individual is not a candidate for bariatric surgery, pharmacological options are available. There are currently six antiobesity drugs approved by the Food and Drug Administration; other pharmacological options are under development.261 Moreover, for those individuals in whom hypertension persists following front-line weight loss interventions, pharmacological blood pressure control options should be considered, and there are a host of options available.56 As obese individuals generally have a relative volume overload state and often have low plasma renin hypertension,187, 188, 262 they typically respond particularly well to diuretics and calcium entry blocking agents. However, considering the adverse renal effects in the obesity–hypertension phenotype, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may provide renal protection in addition to lowering blood pressure; these agents also produce a 25–30% reduction in the development of type 2 diabetes mellitus and are the best agents to reduce left ventricular hypertrophy, which is highly prevalent in the obesity–hypertension phenotype.263, 264, 265 Although β-adrenergic blockers are not currently considered a first-line treatment for hypertension (except in those with coronary heart disease, heart failure, atrial fibrillation or other conditions helped by beta blocking agents), these agents, especially the vasodilating β-blockers,266 may also be useful in the obesity–hypertension phenotype, particularly given their heightened sympathetic nervous system responses. In summary, managing blood pressure is a key goal and should be achieved through all clinically available means possible.
Individuals presenting with obesity are also at higher risk for obstructive sleep apnoea, a condition that elevates blood pressure and increases cardiovascular risk.56, 267 As such, individuals who are obese should be screened for obstructive sleep apnoea and receive appropriate treatment (that is, continuous positive airway pressure) when identified.267
Furthermore, many of the medications available to treat type 2 diabetes are associated with weight gain and increased fat deposition. For overweight and obese patients with diabetes, however, there are Food and Drug Administration-approved medications that have been associated with weight loss and reduced blood pressure. The effects of these agents on weight, and especially on blood pressure, are rather modest but stand in contrast to the gain in weight frequently seen with insulin, thiazolidinediones and insulin secretagogues.
Conclusions
Obesity-related hypertension is an important public health issue. As the prevalence of obesity increases, the prevalence of hypertension with its associated cardiovascular risk will increase as well. Although primary and even primordial prevention is the long-term goal for diminishing the prevalence of obesity, control of both obesity and hypertension in the population at risk is the overriding current challenge. Treating hypertension in the obese requires addressing the obesity as part of the therapeutic plan. Lifestyle management is required in every case, with a focus on weight loss and risk reduction. Some have likened the treatment of obesity with caloric restriction alone to the treatment of hypertension with sodium restriction: it works if extreme enough, but it is not a feasible long-term strategy. In most patients, additional therapies, including medications, aggressive diet counseling and behavioral techniques, and sometimes bariatric surgery will be required.
Obesity and hypertension are two of the most significant health burdens in the world today, resulting in increased risk for noncommunicable diseases and associated adverse cardiovascular events, as well as resulting in trillions of dollars in direct and indirect costs annually. The obesity–hypertension phenotype is common and is associated with a complex cascade of pathophysiological adaptations to multiple systems. When these conditions are combined, there is an increased risk for adverse events and poor outcome; unfortunately, the prevalence of the obesity–hypertension phenotype is high. As such, aggressive treatment of this condition is imperative. Primordial prevention of obesity must become an integral component moving forward and requires a collaborative approach by key stakeholders across multiple sectors; promoting healthy lifestyle behaviors across the lifespan is a key component of primordial prevention and healthy lifestyle medicine; these healthy lifestyle interventions are also a primary intervention in all individuals presenting with the obesity–hypertension phenotype. Surgical and pharmacological options should also be considered in eligible patients. The overarching goals are long-term maintenance of weight loss, blood pressure control and reversal of the pathophysiological cascade associated with this condition.
Finally, even if at the population level, it is clear that obesity is an established risk factor for the development of cardiovascular diseases such as hypertension, diabetes mellitus, coronary heart disease and heart failure, the possible existence of a metabolically healthy obese phenotype (which could be more appropriately be referred to as a lower-risk form of obesity), the important role of regional body fat distribution and ectopic fat accumulation and the presence of an obesity and/or BMI paradox in patients with coronary heart disease are all observations that emphasize the remarkable heterogeneity of obesity. At the population level, these complex obesity-related issues have remained a notable challenge for clinicians dealing with numerous obese phenotypes, and as a consequence, although it is clear that at the population level there is a strong link between the BMI and the incidence of various clinical outcomes, whether reducing the BMI should be the primary target at the clinical level remains debated. It has rather been suggested that targeting key behaviors such as improving nutritional quality and improving cardiorespiratory fitness through regular physical activity would be legitimate approaches contributing to generate ‘healthy weight loss modalities’ as recently suggested by Després.131 Further investigations should consider both metabolic risk and cardiorespiratory fitness in addition to the selection of appropriate obesity index to better identify and manage patients who are ‘at risk’ for the development of cardiovascular diseases.
References
Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M, Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index). National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011; 377: 557–567.
Bastien M, Poirier P, Lemieux I, Després JP . Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis 2014; 56: 369–381.
Fox CS, Pencina MJ, Wilson PWF, Paynter NP, Vasan RS, D’Agostino RB . Lifetime risk of cardiovascular disease among individuals with and without diabetes stratified by obesity status in the Framingham Heart Study. Diabetes Care 2008; 31: 1582–1584.
Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D, Sowers J . Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment: a position paper of The Obesity Society and the American Society of Hypertension. J Clin Hypertens (Greenwich) 2013; 15: 14–33.
Abdullah A, Amin FA, Stoelwinder J, Tanamas SK, Wolfe R, Barendregt J, Peeters A . Estimating the risk of cardiovascular disease using an obese-years metric. BMJ Open 2014; 4: e005629.
Merlotti C, Morabito A, Pontiroli AE . Prevention of type 2 diabetes; a systematic review and meta-analysis of different intervention strategies. Diabetes Obes Metab 2014; 16: 719–727.
Murray CJ, Richards MA, Newton JN, Fenton KA, Anderson HR, Atkinson C, Bennett D, Bernabé E, Blencowe H, Bourne R, Braithwaite T, Brayne C, Bruce NG, Brugha TS, Burney P, Dherani M, Dolk H, Edmond K, Ezzati M, Flaxman AD, Fleming TD, Freedman G, Gunnell D, Hay RJ, Hutchings SJ, Ohno SL, Lozano R, Lyons RA, Marcenes W, Naghavi M, Newton CR, Pearce N, Pope D, Rushton L, Salomon JA, Shibuya K, Vos T, Wang H, Williams HC, Woolf AD, Lopez AD, Davis A . UK health performance: findings of the Global Burden of Disease Study 2010. Lancet 2013; 381: 997–1020.
Falaschetti E, Mindell J, Knott C, Poulter N . Hypertension management in England: a serial cross-sectional study from 1994 to 2011. Lancet 2014; 383: 1912–1919.
Chopra I, Kamal KM, Candrilli SD . Variations in blood pressure and lipid goal attainment in primary care. Curr Med Res Opin 2013; 29: 1115–1125.
Bramlage P, Pittrow D, Wittchen HU, Kirch W, Boehler S, Lehnert H, Hoefler M, Unger T, Sharma AM . Hypertension in overweight and obese primary care patients is highly prevalent and poorly controlled. Am J Hypertens 2004; 17: 904–910.
Banegas JR, López-García E, Dallongeville J, Guallar E, Halcox JP, Borghi C, Massó-González EL, Jiménez FJ, Perk J, Steg PG, De Backer G, Rodríguez-Artalejo F . Achievement of treatment goals for primary prevention of cardiovascular disease in clinical practice across Europe: the EURIKA study. Eur Heart J 2011; 32: 2143–2152.
Zhou L, Li Y, Guo M, Wu Y, Zhao L . Relations of body weight status in early adulthood and weight changes until middle age with hypertension in the Chinese population. Hypertens Res 2016; 39: 913–918.
Wagner KH, Brath H . A global view on the development of non communicable diseases. Prev Med 2012; 54: S38–S41.
Thomas B, Gostin LO . Tackling the global NCD crisis: innovations in law and governance. J Law Med Ethics 2013; 41: 16–27.
Roura LC, Arulkumaran SS . Facing the noncommunicable disease (NCD) global epidemic—the battle of prevention starts in utero—the FIGO challenge. Best Pract Res Clin Obstet Gynaecol 2015; 29: 5–14.
Matheson GO, Klügl M, Engebretsen L, Bendiksen F, Blair SN, Börjesson M, Budgett R, Derman W, Erdener U, Ioannidis JP, Khan KM, Martinez R, Van Mechelen W, Mountjoy M, Sallis RE, Schwellnus M, Shultz R, Soligard T, Steffen K, Sundberg CJ, Weiler R, Ljungqvist A . Prevention and management of noncommunicable disease: the IOC consensus statement, Lausanne 2013. Br J Sports Med 2013; 47: 1003–1011.
Arena R, Guazzi M, Lianov L, Whitsel L, Berra K, Lavie CJ, Kaminsky L, Williams M, Hivert MF, Franklin NC, Myers J, Dengel D, Lloyd-Jones DM, Pinto FJ, Cosentino F, Halle M, Gielen S, Dendale P, Niebauer J, Pelliccia A, Giannuzzi P, Corra U, Piepoli MF, Guthrie G, Shurney D . Healthy lifestyle interventions to combat noncommunicable disease—a novel nonhierarchical connectivity model for key stakeholders: a policy statement from the American Heart Association, European Society of Cardiology, European Association for Cardiovascular Prevention and Rehabilitation, and American College of Preventive Medicine. Mayo Clin Proc 2015; 90: 1082–1103.
American Heart Association. Overweight and Obesity Statistics—2009 Update 2009.
Flegal KM, Carroll MD, Ogden CL, Curtin LR . Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010; 303: 235–241.
Yang L, Colditz GA . Prevalence of overweight and obesity in the United States, 2007-2012. JAMA Intern Med 2015; 175: 1412–1413.
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015; 131: e29–322.
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E . Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384: 766–781.
Masters RK, Reither EN, Powers DA, Yang YC, Burger AE, Link BG . The impact of obesity on US mortality levels: the importance of age and cohort factors in population estimates. Am J Public Health 2013; 103: 1895–1901.
Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA . A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2224–2260.
Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD, American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation 2010; 121: 586–613.
Caldarone E, Lombardi M, Severi P, Leggio M . Ambient air pollution and hypertension: a relationship that strikes around the clock. Arch Clin Hypertens 2016; 1: 044–045.
Poulter NR, Prabhakaran D, Caulfield M . Hypertension. Lancet 2015; 386: 801–812.
Kotchen TA . Obesity-related hypertension: epidemiology, pathophysiology, and clinical management. Am J Hypertens 2010; 23: 1170–1178.
Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G . Mechanisms of obesity-induced hypertension. Hypertens Res 2010; 33: 386–393.
Katz DL . Perspective: obesity is not a disease. Nature. 2014; 508: S57.
Després JP . Excess visceral adipose tissue/ectopic fat the missing link in the obesity paradox? J Am Coll Cardiol 2011; 57: 1887–1889.
Nazare JA, Smith J, Borel AL, Aschner P, Barter P, Van Gaal L, Tan CE, Wittchen HU, Matsuzawa Y, Kadowaki T, Ross R, Brulle-Wohlhueter C, Alméras N, Haffner SM, Balkau B, Després JP, INSPIRE ME IAA Investigators. Usefulness of measuring both body mass index and waist circumference for the estimation of visceral adiposity and related cardiometabolic risk profile (from the INSPIRE ME IAA study). Am J Cardiol 2015; 115: 307–315.
WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363: 157–163.
Jee SH, Sull JW, Park J, Lee SY, Ohrr H, Guallar E, Samet JM . Body-mass index and mortality in Korean men and women. N Engl J Med 2006; 355: 779–787.
Gu D, He J, Duan X, Reynolds K, Wu X, Chen J, Huang G, Chen CS, Whelton PK . Body weight and mortality among men and women in China. JAMA 2006; 295: 776–783.
Zheng W, McLerran DF, Rolland B, Zhang X, Inoue M, Matsuo K, He J, Gupta PC, Ramadas K, Tsugane S, Irie F, Tamakoshi A, Gao YT, Wang R, Shu XO, Tsuji I, Kuriyama S, Tanaka H, Satoh H, Chen CJ, Yuan JM, Yoo KY, Ahsan H, Pan WH, Gu D, Pednekar MS, Sauvaget C, Sasazuki S, Sairenchi T, Yang G, Xiang YB, Nagai M, Suzuki T, Nishino Y, You SL, Koh WP, Park SK, Chen Y, Shen CY, Thornquist M, Feng Z, Kang D, Boffetta P, Potter JD . Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med 2011; 364: 719–729.
Karelis AD, Faraj M, Bastard JP, St-Pierre DH, Brochu M, Prud'homme D, Rabasa-Lhoret R . The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab 2005; 90: 4145–4150.
Gruberg L, Weissman NJ, Waksman R, Fuchs S, Deible R, Pinnow EE, Ahmed LM, Kent KM, Pichard AD, Suddath WO, Satler LF, Lindsay J Jr. . The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol 2002; 39: 578–584.
Lemieux I . Energy partitioning in gluteal-femoral fat: does the metabolic fate of triglycerides affect coronary heart disease risk? Arterioscler Thromb Vasc Biol 2004; 24: 795–797.
Després JP, Lemieux I . Abdominal obesity and metabolic syndrome. Nature 2006; 444: 881–887.
Després JP . Body fat distribution and risk of cardiovascular disease: an update. Circulation 2012; 126: 1301–1313.
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ . Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003; 348: 1625–1638.
Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P Jr, Razak F, Sharma AM, Anand SS . INTERHEART Study Investigators. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet 2005; 366: 1640–1649.
Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF . Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med 2006; 355: 763–778.
Kannel WB, Brand N, Skinner JJ Jr, Dawber TR, McNamara PM . The relation of adiposity to blood pressure and development of hypertension. The Framingham study. Ann Intern Med 1967; 67: 48–59.
Vague J . The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr 1956; 4: 20–34.
Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjöström L . Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J (Clin Res Ed) 1984; 289: 1257–1261.
Larsson B, Svärdsudd K, Welin L, Wilhelmsen L, Björntorp P, Tibblin G . Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed) 1984; 288: 1401–1404.
Cassano PA, Segal MR, Vokonas PS, Weiss ST . Body fat distribution, blood pressure, and hypertension. A prospective cohort study of men in the normative aging study. Ann Epidemiol 1990; 1: 33–48.
Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK, Adams PW . Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab 1982; 54: 254–260.
Krotkiewski M, Bjorntorp P, Sjostrom L, Smith U . Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest 1983; 72: 1150–1162.
Kalkhoff RK, Hartz AH, Rupley D, Kissebah AH, Kelber S . Relationship of body fat distribution to blood pressure, carbohydrate tolerance, and plasma lipids in healthy obese women. J Lab Clin Med 1983; 102: 621–627.
Ferrannini E, Buzzigoli G, Bonadonna R, Giorico MA, Oleggini M, Graziadei L, Pedrinelli R, Brandi L, Bevilacqua S . Insulin resistance in essential hypertension. N Engl J Med 1987; 317: 350–357.
Modan M, Halkin H, Almog S, Lusky A, Eshkol A, Shefi M, Shitrit A, Fuchs Z . Hyperinsulinemia. A link between hypertension obesity and glucose intolerance. J Clin Invest 1985; 75: 809–817.
Landsberg L . In: Birkenager WH, Robertson JIS, Zanchetti A (eds). Handbook of Hypertension. Hypertension in the Twentieth Century: Concepts and Achievements vol. 22. Elsevier: Amsterdam, The Netherlands. 2004, 245–261.
Reisin E, Jack AV . Obesity and hypertension: mechanisms, cardio-renal consequences, and therapeutic approaches. Med Clin North Am 2009; 93: 733–751.
Narkiewicz K . Obesity, hypertension—the issue is more complex than we thought. Nephrol Dial Transplant 2006; 21: 264–267.
Wirix AJ, Kaspers PJ, Nauta J, Chinapaw MJ, Kist-van Holthe JE . Pathophysiology of hypertension in obese children: a systematic review. Obes Rev 2015; 16: 831–842.
Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME . Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res 2015; 116: 991–1006.
Lambert EA, Straznicky NE, Dixon JB, Lambert GW . Should the sympathetic nervous system be a target to improve cardiometabolic risk in obesity? Am J Physiol Heart Circ Physiol 2015; 309: H244–H258.
Alvarez GE, Beske SD, Ballard TP, Davy KP . Sympathetic neural activation in visceral obesity. Circulation 2002; 106: 2533–2536.
Thorp AA, Schlaich MP . Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. J Diabetes Res 2015; 2015: 341583.
Han SH, Quon MJ, Kim JA, Koh KK . Adiponectin and cardiovascular disease: response to therapeutic interventions. J Am Coll Cardiol 2007; 49: 531–538.
Lim S, Quon MJ, Koh KK . Modulation of adiponectin as a potential therapeutic strategy. Atherosclerosis 2014; 233: 721–728.
Matsuzawa Y, Funahashi T, Kihara S, Shimomura I . Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol 2004; 24: 29–33.
Koh KK, Park SM, Quon MJ . Leptin and cardiovascular disease: response to therapeutic interventions. Circulation 2008; 117: 3238–3249.
Quercioli A, Pataky Z, Montecucco F, Carballo S, Thomas A, Staub C, Di Marzo V, Vincenti G, Ambrosio G, Ratib O, Golay A, Mach F, Harsch E, Schindler TH . Coronary vasomotor control in obesity and morbid obesity: contrasting flow responses with endocannabinoids, leptin, and inflammation. JACC Cardiovasc Imaging 2012; 5: 805–815.
Kennedy A, Gettys TW, Watson P, Wallace P, Ganaway E, Pan Q, Garvey WT . The metabolic significance of leptin in humans: gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J Clin Endocrinol Metab 1997; 82: 1293–1300.
Tang-Christensen M, Havel PJ, Jacobs RR, Larsen PJ, Cameron JL . Central administration of leptin inhibits food intake and activates the sympathetic nervous system in rhesus macaques. J Clin Endocrinol Metab 1999; 84: 711–717.
Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI . Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest 1997; 100: 270–278.
Rowe JW, Young JB, Minaker KL, Stevens AL, Pallotta J, Landsberg L . Effect of insulin and glucose infusions on sympathetic nervous system activity in normal man. Diabetes 1981; 30: 219–225.
Hausberg M, Mark AL, Hoffman RP, Sinkey CA, Anderson EA . Dissociation of sympathoexcitatory and vasodilator actions of modestly elevated plasma insulin levels. J Hypertens 1995; 13: 1015–1021.
Troisi RJ, Weiss ST, Parker DR, Sparrow D, Young JB, Landsberg L . Relation of obesity and diet to sympathetic nervous system activity. Hypertension 1991; 17: 669–677.
Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, Giannattasio C, Brunani A, Cavagnini F, Mancia G . Sympathetic activation in obese normotensive subjects. Hypertension 1995; 25: 560–563.
Scherrer U, Randin D, Tappy L, Vollenweider P, Jéquier E, Nicod P . Body fat and sympathetic nerve activity in healthy subjects. Circulation 1994; 89: 2634–2640.
Grassi G, Seravalle G, Colombo M, Bolla G, Cattaneo BM, Cavagnini F, Mancia G . Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation 1998; 97: 2037–2042.
DeFronzo RA . Insulin and renal sodium handling: clinical implications. Int J Obes 1981; 5 (Suppl 1): 93–104.
Heyman E, Gamelin FX, Aucouturier J, Di Marzo V . The role of the endocannabinoid system in skeletal muscle and metabolic adaptations to exercise: potential implications for the treatment of obesity. Obes Rev 2012; 13: 1110–1124.
Di Marzo V, Côté M, Matias I, Lemieux I, Arsenault BJ, Cartier A, Piscitelli F, Petrosino S, Alméras N, Després JP . Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: associations with changes in metabolic risk factors. Diabetologia 2009; 52: 213–217.
Naguib MT . Kidney disease in the obese patient. South Med J 2014; 107: 481–485.
Snyder S, Turner GA, Turner A . Obesity-related kidney disease. Prim Care 2014; 41: 875–893.
Ahmed SB, Fisher ND, Stevanovic R, Hollenberg NK . Body mass index and angiotensin-dependent control of the renal circulation in healthy humans. Hypertension 2005; 46: 1316–1320.
Hall JE, Guyton AC, Coleman TG, Mizelle HL, Woods LL . Regulation of arterial pressure: role of pressure natriuresis and diuresis. Fed Proc 1986; 45: 2897–2903.
Rocchini AP, Key J, Bondie D, Chico R, Moorehead C, Katch V, Martin M . The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N Engl J Med 1989; 321: 580–585.
Goodfriend TL . Obesity, sleep apnea, aldosterone, and hypertension. Curr Hypertens Rep 2008; 10: 222–226.
Sarzani R, Salvi F, Dessi-Fulgheri P, Rappelli A . Renin-angiotensin system, natriuretic peptides, obesity, metabolic syndrome, and hypertension: an integrated view in humans. J Hypertens 2008; 26: 831–843.
Bomback AS, Klemmer PJ . Interaction of aldosterone and extracellular volume in the pathogenesis of obesity-associated kidney disease: a narrative review. Am J Nephrol 2009; 30: 140–146.
Frigolet ME, Torres N, Tovar AR . The renin-angiotensin system in adipose tissue and its metabolic consequences during obesity. J Nutr Biochem 2013; 24: 2003–2015.
Adamczak M, Wiecek A, Funahashi T, Chudek J, Kokot F, Matsuzawa Y . Decreased plasma adiponectin concentration in patients with essential hypertension. Am J Hypertens 2003; 16: 72–75.
Van Cauter SK . Sleep as a mediator of the relationship between socioeconomic status and health: a hypothesis. Ann NY Acad Sci 1999; 896: 254–261.
Narkiewicz K, Somers VK . Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand 2003; 177: 385–390.
Wolk R, Shamsuzzaman AS, Somers VK . Obesity, sleep apnea, and hypertension. Hypertension 2003; 42: 1067–1074.
Spiegel K, Leproult R, L'hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E . Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 2004; 89: 5762–5771.
Barker DJ . Fetal programming of coronary heart disease. Trends Endocrinol Metab 2002; 13: 364–368.
Silverman BL, Landsberg L, Metzger BE . Fetal hyperinsulinism in offspring of diabetic mothers. Association with the subsequent development of childhood obesity. Ann NY Acad Sci 1993; 699: 36–45.
Hausberg M, Barenbrock M, Kosch M . Elevated sympathetic nerve activity: the link between low birth size and adult-onset metabolic syndrome? J Hypertens 2004; 22: 1087–1089.
Caballero AE . Endothelial dysfunction in obesity and insulin resistance: a road to diabetes and heart disease. Obes Res 2003; 11: 1278–1289.
Barrett EJ, Eggleston EM, Inyard AC, Wang H, Li G, Chai W, Liu Z . The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 2009; 52: 752–764.
Kappus RM, Fahs CA, Smith D, Horn GP, Agiovlasitis S, Rossow L, Jae SY, Heffernan KS, Fernhall B . Obesity and overweight associated with increased carotid diameter and decreased arterial function in young otherwise healthy men. Am J Hypertens 2014; 27: 628–634.
Wofford MR, Smith G, Minor DS . The treatment of hypertension in obese patients. Curr Hypertens Rep 2008; 10: 143–150.
Colosia AD, Palencia R, Khan S . Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabetes Metab Syndr Obes 2013; 6: 327–338.
Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J, American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011; 123: e18–e209.
Wang Y, Wang QJ . The prevalence of prehypertension and hypertension among US adults according to the new Joint National Committee guidelines. Arch Intern Med 2004; 164: 2126–2134.
Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB . Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med 2002; 162: 1867–1872.
Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D . Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension 1995; 25: 305–313.
Franklin SS, Gustin W 4th, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D . Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997; 96: 308–315.
Lloyd-Jones DM, Liu K, Colangelo LA, Yan LL, Klein L, Loria CM, Lewis CE, Savage P . Consistently stable or decreased body mass index in young adulthood and longitudinal changes in metabolic syndrome components: the Coronary Artery Risk Development in Young Adults Study. Circulation 2007; 115: 1004–1011.
Mamun AA, Lawlor DA, O'Callaghan MJ, Williams GM, Najman JM . Effect of body mass index changes between ages 5 and 14 on blood pressure at age 14: findings from a birth cohort study. Hypertension 2005; 45: 1083–1087.
Leggio M, Cruciani G, Sgorbini L, Mazza A, Bendini MG, Pugliese M, Leggio F, Jesi AP . Obesity-related adjunctive systo-diastolic ventricular dysfunction in patients with hypertension: echocardiographic assessment with tissue Doppler velocity and strain imaging. Hypertens Res 2011; 34: 468–473.
Stamler J, Dyer AR, Shekelle RB, Neaton J, Stamler R . Relationship of baseline major risk factors to coronary and all-cause mortality, and to longevity: findings from long-term follow-up of Chicago cohorts. Cardiology 1993; 82: 191–222.
Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M . Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339: 229–234.
Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M . Type 2 diabetes as a ‘coronary heart disease equivalent’: an 18-year prospective population-based study in Finnish subjects. Diabetes Care 2005; 28: 2901–2907.
Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Häring HU . Identification and characterization of metabolically benign obesity in humans. Arch Intern Med 2008; 168: 1609–1616.
Aguilar-Salinas CA, García EG, Robles L, Riaño D, Ruiz-Gomez DG, García-Ulloa AC, Melgarejo MA, Zamora M, Guillen-Pineda LE, Mehta R, Canizales-Quinteros S, Tusie Luna MT, Gómez-Pérez FJ . High adiponectin concentrations are associated with the metabolically healthy obese phenotype. J Clin Endocrinol Metab 2008; 93: 4075–4079.
Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D’Agostino RB . Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab 2006; 91: 2906–2912.
Karelis AD, Messier V, Brochu M, Rabasa-Lhoret R . Metabolically healthy but obese women: effect of an energy-restricted diet. Diabetologia 2008; 51: 1752–1754.
Arnlöv J, Ingelsson E, Sundström J, Lind L . Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation 2010; 121: 230–236.
Khan UI, Wang D, Thurston RC, Sowers M, Sutton-Tyrrell K, Matthews KA, Barinas-Mitchell E, Wildman RP . Burden of subclinical cardiovascular disease in ‘metabolically benign’ and ‘at-risk’ overweight and obese women: the Study of Women’s Health Across the Nation (SWAN). Atherosclerosis 2011; 217: 179–186.
Chang Y, Kim BK, Yun KE, Cho J, Zhang Y, Rampal S, Zhao D, Jung HS, Choi Y, Ahn J, Lima JA, Shin H, Guallar E, Ryu S . Metabolically-healthy obesity and coronary artery calcification. J Am Coll Cardiol 2014; 63: 2679–2686.
Park J, Kim SH, Cho GY, Baik I, Kim NH, Lim HE, Kim EJ, Park CG, Lim SY, Kim YH, Kim H, Lee SK, Shin C . Obesity phenotype and cardiovascular changes. J Hypertens 2011; 29: 1765–1772.
Lind L, Siegbahn A, Ingelsson E, Sundström J, Arnlöv J . A detailed cardiovascular characterization of obesity without the metabolic syndrome. Arterioscler Thromb Vasc Biol 2011; 31: e27–e34.
Hinnouho GM, Czernichow S, Dugravot A, Batty GD, Kivimaki M, Singh-Manoux A . Metabolically healthy obesity and risk of mortality: does the definition of metabolic health matter? Diabetes Care 2013; 36: 2294–2300.
Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR . The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med 2008; 168: 1617–1624.
Velho S, Paccaud F, Waeber G, Vollenweider P, Marques-Vidal P . Metabolically healthy obesity: different prevalences using different criteria. Eur J Clin Nutr 2010; 64: 1043–1051.
Lee SK, Kim SH, Cho GY, Baik I, Lim HE, Park CG, Lee JB, Kim YH, Lim SY, Kim H, Shin C . Obesity phenotype and incident hypertension: a prospective community-based cohort study. J Hypertens 2013; 31: 145–151.
Kramer CK, Zinman B, Retnakaran R . Are metabolically healthy overweight and obesity benign conditions?: a systematic review and meta-analysis. Ann Intern Med 2013; 159: 758–769.
Fan J, Song Y, Chen Y, Hui R, Zhang W . Combined effect of obesity and cardiometabolic abnormality on the risk of cardiovascular disease: a meta-analysis of prospective cohort studies. Int J Cardiol 2013; 168: 4761–4768.
Puri R . Is it finally time to dispel the concept of metabolically-healthy obesity? J Am Coll Cardiol 2014; 63: 2687–2688.
Ortega FB, Lee DC, Katzmarzyk PT, Ruiz JR, Sui X, Church TS, Blair SN . The intriguing metabolically healthy but obese phenotype: cardiovascular prognosis and role of fitness. Eur Heart J 2013; 34: 389–397.
Arsenault BJ, Lachance D, Lemieux I, Alméras N, Tremblay A, Bouchard C, Pérusse L, Després JP . Visceral adipose tissue accumulation, cardiorespiratory fitness, and features of the metabolic syndrome. Arch Intern Med 2007; 167: 1518–1525.
Després JP . Obesity and cardiovascular disease: weight loss is not the only target. Can J Cardiol 2015; 31: 216–222.
Uretsky S, Messerli FH, Bangalore S, Champion A, Cooper-Dehoff RM, Zhou Q, Pepine CJ . Obesity paradox in patients with hypertension and coronary artery disease. Am J Med 2007; 120: 863–870.
Wassertheil-Smoller S, Fann C, Allman RM, Black HR, Camel GH, Davis B, Masaki K, Pressel S, Prineas RJ, Stamler J, Vogt TM . Relation of low body mass to death and stroke in the systolic hypertension in the elderly program. The SHEP Cooperative Research Group. Arch Intern Med 2000; 160: 494–500.
Lavie CJ, Milani RV, Ventura HO . Obesity, heart disease, and favorable prognosis—truth or paradox? Am J Med 2007; 120: 825–826.
von Haehling S, Doehner W, Anker SD . Revisiting the obesity paradox in heart failure: new insights? Eur J Heart Fail 2011; 13: 130–132.
Flegal KM, Kit BK, Orpana H, Graubard BI . Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013; 309: 71–82.
Shah RV, Abbasi SA, Yamal JM, Davis BR, Barzilay J, Einhorn PT, Goldfine AB, ALLHAT Collaborative Research Group. Impaired fasting glucose and body mass index as determinants of mortality in ALLHAT: is the obesity paradox real? J Clin Hypertens (Greenwich) 2014; 16: 451–458.
Lavie CJ, De Schutter A, Milani RV . Healthy obese versus unhealthy lean: the obesity paradox. Nat Rev Endocrinol 2015; 11: 55–62.
Khalid U, Ather S, Bavishi C, Chan W, Loehr LR, Wruck LM, Rosamond WD, Chang PP, Coresh J, Virani SS, Nambi V, Bozkurt B, Ballantyne CM, Deswal A . Premorbid body mass index and mortality after incident heart failure: the ARIC Study. J Am Coll Cardiol 2014; 64: 2743–2749.
Hansen BC, Bray GA . The Metabolic Syndrome Epidemiology, Clinical Treatment, and Underlying Mechanisms. Humana Press: Totowa, NJ, USA. 2008.
Reaven GM . In: Hansen BC, Bray GA eds. The Metabolic Syndrome Epidemiology, Clinical Treatment, and Underlying Mechanisms. Humana Press: Totowa, NJ, USA. 2008, 11–36.
Ervin R . Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Rep 2009; 13: 1–8.
Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C, American Heart Association National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004; 109: 433–438.
Sánchez-Lozada LG, Tapia E, Jiménez A, Bautista P, Cristóbal M, Nepomuceno T, Soto V, Avila-Casado C, Nakagawa T, Johnson RJ, Herrera-Acosta J, Franco M . Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Renal Physiol 2007; 292: F423–F429.
Lee JE, Kim YG, Choi YH, Huh W, Kim DJ, Oh HY . Serum uric acid is associated with microalbuminuria in prehypertension. Hypertension 2006; 47: 962–967.
Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, Gersch MS, Benner S, Sánchez-Lozada LG . Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 2007; 86: 899–906.
Choi J . Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum 2008; 59: 109–116.
Spiegel K, Leproult R, Van Cauter E . Impact of sleep debt on metabolic and endocrine function. Lancet 1999; 354: 1435–1439.
Bass J, Turek FW . Sleepless in America: a pathway to obesity and the metabolic syndrome? Arch Intern Med 2005; 165: 15–16.
Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J . Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005; 308: 1043–1045.
Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD . Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med 2010; 153: 435–441.
Egan BM, Li J, Hutchison FN, Ferdinand KC . Hypertension in the United States, 1999 to 2012: progress toward Healthy People 2020 goals. Circulation 2014; 130: 1692–1699.
Borlaug BA . The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2014; 11: 507–515.
Peng J, Zhao Y, Zhang H, Liu Z, Wang Z, Tang M, Zhong M, Lu F, Zhang W . Prevention of metabolic disorders with telmisartan and indapamide in a Chinese population with high-normal blood pressure. Hypertens Res 2015; 38: 123–131.
Janghorbani M, Bonnet F, Amini M . Glucose and the risk of hypertension in first-degree relatives of patients with type 2 diabetes. Hypertens Res 2015; 38: 349–354.
Fujii M, Ohnishi H, Saitoh S, Akasaka H, Miura T, Mori M . The combination of abdominal obesity and high-sensitivity C-reactive protein predicts new-onset hypertension in the general Japanese population: the Tanno-Sobetsu study. Hypertens Res 2015; 38: 426–432.
Nagai M, Ohkubo T, Murakami Y, Takashima N, Kadota A, Miyagawa N, Saito Y, Nishi N, Okuda N, Kiyohara Y, Nakagawa H, Nakamura Y, Fujiyoshi A, Abbott RD, Okamura T, Okayama A, Ueshima H, Miura K, NIPPON DATA80/90/2010 Research Group. Secular trends of the impact of overweight and obesity on hypertension in Japan, 1980-2010. Hypertens Res 2015; 38: 790–795.
Chen SC, Lee WH, Hsu PC, Huang JC, Lee CS, Lin TH, Voon WC, Lai WT, Sheu SH, Su HM . Association of body mass index and left ventricular mass index with abnormally low and high ankle-brachial indices in chronic kidney disease. Hypertens Res 2016; 39: 166–170.
Hirata C, Miyai N, Idoue A, Utsumi M, Hattori S, Iwahara A, Uematsu Y, Shiba M, Arita M . Effect of metabolic syndrome components and their clustering on carotid atherosclerosis in a sample of the general Japanese population. Hypertens Res 2016; 39: 362–366.
Dimitriadis K, Tsioufis C, Mazaraki A, Liatakis I, Koutra E, Kordalis A, Kasiakogias A, Flessas D, Tentolouris N, Tousoulis D . Waist circumference compared with other obesity parameters as determinants of coronary artery disease in essential hypertension: a 6-year follow-up study. Hypertens Res 2016; 39: 475–479.
Ye C, Pan Y, Xu X, Su S, Snieder H, Treiber F, Kapuku G, Wang X . Pulse wave velocity in elastic and muscular arteries: tracking stability and association with anthropometric and hemodynamic measurements. Hypertens Res 2016; 39: 786–791.
l’Allemand-Jander D . Clinical diagnosis of metabolic and cardiovascular risks in overweight children: early development of chronic diseases in the obese child. Int J Obes (Lond) 2010; 34: S32–S36.
Srinivasan SR, Myers L, Berenson GS . Changes in metabolic syndrome variables since childhood in prehypertensive and hypertensive subjects: the Bogalusa Heart Study. Hypertension 2006; 48: 33–39.
Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W, Sun C, Cheung M, Viikari JS, Dwyer T, Raitakari OT . Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med 2011; 365: 1876–1885.
Craigie AM, Lake AA, Kelly SA, Adamson AJ, Mathers JC . Tracking of obesity-related behaviours from childhood to adulthood: a systematic review. Maturitas 2011; 70: 266–284.
Eriksson J, Forsén T, Tuomilehto J, Osmond C, Barker D . Fetal and childhood growth and hypertension in adult life. Hypertension 2000; 36: 790–794.
Huang RC, Mori TA, Burke V, Newnham J, Stanley FJ, Landau LI, Kendall GE, Oddy WH, Beilin LJ . Synergy between adiposity, insulin resistance, metabolic risk factors, and inflammation in adolescents. Diabetes Care 2009; 32: 695–701.
Huang RC, Burke V, Newnham JP, Stanley FJ, Kendall GE, Landau LI, Oddy WH, Blake KV, Palmer LJ, Beilin LJ . Perinatal and childhood origins of cardiovascular disease. Int J Obes (Lond) 2007; 31: 236–244.
Chivers P, Hands B, Parker H, Beilin L, Kendall G, Bulsara M . Longitudinal modelling of body mass index from birth to 14 years. Obes Facts 2009; 2: 302–310.
Waters E, de Silva-Sanigorski A, Hall BJ, Brown T, Campbell KJ, Gao Y, Armstrong R, Prosser L, Summerbell CD . Interventions for preventing obesity in children. Cochrane Database Syst Rev 2011; (12 CD001871.
Vandongen R, Jenner DA, Thompson C, Taggart AC, Spickett EE, Burke V, Beilin LJ, Milligan RA, Dunbar DL . A controlled evaluation of a fitness and nutrition intervention program on cardiovascular health in 10- to 12-year-old children. Prev Med 1995; 24: 9–22.
Burke V, Milligan RA, Thompson C, Taggart AC, Dunbar DL, Spencer MJ, Medland A, Gracey MP, Vandongen R, Beilin LJ . A controlled trial of health promotion programs in 11-year-olds using physical activity ‘enrichment’ for higher risk children. J Pediatr 1998; 132: 840–848.
Milligan RA, Thompson C, Vandongen R, Beilin LJ, Burke V . Clustering of cardiovascular risk factors in Australian adolescents: association with dietary excesses and deficiencies. J Cardiovasc Risk 1995; 2: 515–523.
Taylor BJ, Heath AL, Galland BC, Gray AR, Lawrence JA, Sayers RM, Dale K, Coppell KJ, Taylor RW . Prevention of Overweight in Infancy (POI.nz) study: a randomised controlled trial of sleep, food and activity interventions for preventing overweight from birth. BMC Public Health 2011; 11: 942.
Berenson GS . Cardiovascular health promotion for children: a model for a Parish (County)-wide program (implementation and preliminary results). Prev Cardiol 2010; 13: 23–28.
Sobko T, Svensson V, Ek A, Ekstedt M, Karlsson H, Johansson E, Cao Y, Hagströmer M, Marcus C . A randomised controlled trial for overweight and obese parents to prevent childhood obesity—Early STOPP (STockholm Obesity Prevention Program). BMC Public Health 2011; 11: 336.
Christie D, Hudson L, Mathiot A, Cole TJ, Karlsen S, Kessel A, Kinra S, Morris S, Nazareth I, Sovio U, Wong IC, Viner RM . Assessing the efficacy of the Healthy Eating and Lifestyle Programme (HELP) compared with enhanced standard care of the obese adolescent in the community: study protocol for a randomized controlled trial. Trials 2011; 12: 242.
Borys JM, Le Bodo Y, Jebb SA, Seidell JC, Summerbell C, Richard D, De Henauw S, Moreno LA, Romon M, Visscher TL, Raffin S, Swinburn B, EEN Study Group. EPODE approach for childhood obesity prevention: methods, progress and international development. Obes Rev 2012; 13: 299–315.
Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB . Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011; 364: 2392–2404.
Wang Y, Beydoun MA . The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev 2007; 29: 6–28.
Popkin BM, Adair LS, Ng SW . Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 2011; 70: 3–21.
Zhai F, Wang H, Wang Z, Popkin BM, Chen C . Closing the energy gap to prevent weight gain in China. Obes Rev 2008; 9 (Suppl 1): 107–112.
Akers JD, Estabrooks PA, Davy BM . Translational research: bridging the gap between long-term weight loss maintenance research and practice. J Am Diet Assoc 2010; 110: 1511–1522 1522.e1–1522.e3.
Anderson SE, Whitaker RC . Prevalence of obesity among US preschool children in different racial and ethnic groups. Arch Pediatr Adolesc Med 2009; 163: 344–348.
Anand SS, Yusuf S . Stemming the global tsunami of cardiovascular disease. Lancet 2011; 377: 529–532.
Leggio M, Mazza A, Cruciani G, Sgorbini L, Pugliese M, Bendini MG, Severi P, Jesi AP . Effects of exercise training on systo-diastolic ventricular dysfunction in patients with hypertension: an echocardiographic study with tissue velocity and strain imaging evaluation. Hypertens Res 2014; 37: 649–654.
Liu J, Sui X, Lavie CJ, Zhou H, Park YM, Cai B, Liu J, Blair SN . Effects of cardiorespiratory fitness on blood pressure trajectory with aging in a cohort of healthy men. J Am Coll Cardiol 2014; 64: 1245–1253.
Lavie CJ, McAuley PA, Church TS, Milani RV, Blair SN . Obesity and cardiovascular diseases: implications regarding fitness, fatness, and severity in the obesity paradox. J Am Coll Cardiol 2014; 63: 1345–1354.
Schwingshackl L, Dias S, Hoffmann G . Impact of long-term lifestyle programmes on weight loss and cardiovascular risk factors in overweight/obese participants: a systematic review and network meta-analysis. Syst Rev 2014; 3: 130.
Nurkkala M, Kaikkonen K, Vanhala ML, Karhunen L, Keränen AM, Korpelainen R . Lifestyle intervention has a beneficial effect on eating behavior and long-term weight loss in obese adults. Eat Behav 2015; 18: 179–185.
Newton RL, Griffith DM, Kearney WB, Bennett GG . A systematic review of weight loss, physical activity and dietary interventions involving African American men. Obes Rev 2014; 15: 93–106.
Clark JE . Diet, exercise or diet with exercise: comparing the effectiveness of treatment options for weight-loss and changes in fitness for adults (18-65 years old) who are overfat, or obese; systematic review and meta-analysis. J Diabetes Metab Disord 2015; 14: 31.
Ades PA, Savage PD, Harvey-Berino J . The treatment of obesity in cardiac rehabilitation. J Cardiopulm Rehabil Prev 2010; 30: 289–298.
Ades PA, Savage PD, Toth MJ, Harvey-Berino J, Schneider DJ, Bunn JY, Audelin MC, Ludlow M . High-calorie-expenditure exercise: a new approach to cardiac rehabilitation for overweight coronary patients. Circulation 2009; 119: 2671–2678.
Looney SM, Raynor HA . Behavioral lifestyle intervention in the treatment of obesity. Health Serv Insights 2013; 6: 15–31.
Ades PA . A lifestyle program of exercise and weight loss is effective in preventing and treating type 2 diabetes mellitus: why are programs not more available? Prev Med 2015; 80: 50–52.
Spahn JM, Reeves RS, Keim KS, Laquatra I, Kellogg M, Jortberg B, Clark NA . State of the evidence regarding behavior change theories and strategies in nutrition counseling to facilitate health and food behavior change. J Am Diet Assoc 2010; 110: 879–891.
Lucini D, Cesana G, Vigo C, Malacarne M, Pagani M . Reducing weight in an internal medicine outpatient clinic using a lifestyle medicine approach: a proof of concept. Eur J Intern Med 2015; 26: 680–684.
McNellis R, Lewis P 3rd . Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors. Am Fam Physician 2015; 92: 509–510.
Voulgari C, Pagoni S, Vinik A, Poirier P . Exercise improves cardiac autonomic function in obesity and diabetes. Metabolism 2013; 62: 609–621.
de Jonge L, Moreira EA, Martin CK, Ravussin E, Pennington CALERIE Team. Impact of 6-month caloric restriction on autonomic nervous system activity in healthy, overweight, individuals. Obesity (Silver Spring) 2010; 18: 414–416.
Hegde SM, Solomon SD . Influence of physical activity on hypertension and cardiac structure and function. Curr Hypertens Rep 2015; 17: 77.
Severi P, D’Emidio S, Armeni M, Bravi V, Leggio M . Exercise training and hypertension: ready for prime-time? Sports Med Rehabil J 2017; 2: 1011.
Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS . The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis 2014; 56: 441–447.
Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM . Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension 2003; 42: 878–884.
Aucott L, Poobalan A, Smith WC, Avenell A, Jung R, Broom J . Effects of weight loss in overweight/obese individuals and long-term hypertension outcomes: a systematic review. Hypertension 2005; 45: 1035–1041.
Aucott L, Rothnie H, McIntyre L, Thapa M, Waweru C, Gray D . Long-term weight loss from lifestyle intervention benefits blood pressure?: a systematic review. Hypertension 2009; 54: 756–762.
Semlitsch T, Jeitler K, Berghold A, Horvath K, Posch N, Poggenburg S, Siebenhofer A . Long-term effects of weight-reducing diets in people with hypertension. Cochrane Database Syst Rev 2016; 3: CD008274.
Bray GA . Lifestyle and pharmacological approaches to weight loss: efficacy and safety. J Clin Endocrinol Metab 2008; 93 (Suppl 1): S81–S88.
Makris A, Foster GD . Dietary approaches to the treatment of obesity. Psychiatr Clin North Am 2011; 34: 813–827.
Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, Smith WC, Jung RT, Campbell MK, Grant AM . Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess 2004; 8 iii–iv 1–182.
Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS Jr, Brehm BJ, Bucher HC . Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med 2006; 166: 285–293.
Appel LJ . ASH position paper: dietary approaches to lower blood pressure. J Am Soc Hypertens 2009; 3: 321–331.
Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N . A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997; 336: 1117–1124.
Kastorini CM, Milionis HJ, Esposito K, Giugliano D, Goudevenos JA, Panagiotakos DB . The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol 2011; 57: 1299–1313.
Beilin LJ, Burke V . Vegetarian diet components, protein and blood pressure: which nutrients are important? Clin Exp Pharmacol Physiol 1995; 22: 195–198.
Miller ER 3rd, Erlinger TP, Young DR, Jehn M, Charleston J, Rhodes D, Wasan SK, Appel LJ . Results of the Diet, Exercise, and Weight Loss Intervention Trial (DEW-IT). Hypertension 2002; 40: 612–618.
Elmer PJ, Obarzanek E, Vollmer WM, Simons-Morton D, Stevens VJ, Young DR, Lin PH, Champagne C, Harsha DW, Svetkey LP, Ard J, Brantley PJ, Proschan MA, Erlinger TP, Appel LJ, PREMIER Collaborative Research Group. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomized trial. Ann Intern Med 2006; 144: 485–495.
Jehn ML, Patt MR, Appel LJ, Miller ER 3rd . One year follow-up of overweight and obese hypertensive adults following intensive lifestyle therapy. J Hum Nutr Diet 2006; 19: 349–354.
Lien LF, Brown AJ, Ard JD, Loria C, Erlinger TP, Feldstein AC, Lin PH, Champagne CM, King AC, McGuire HL, Stevens VJ, Brantley PJ, Harsha DW, McBurnie MA, Appel LJ, Svetkey LP . Effects of PREMIER lifestyle modifications on participants with and without the metabolic syndrome. Hypertension 2007; 50: 609–616.
Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, Caccia C, Johnson J, Waugh R, Sherwood A . Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med 2010; 170: 126–135.
Gao SK, Fitzpatrick AL, Psaty B, Jiang R, Post W, Cutler J, Maciejewski ML . Suboptimal nutritional intake for hypertension control in 4 ethnic groups. Arch Intern Med 2009; 169: 702–707.
Burke V, Beilin LJ, Cutt HE, Mansour J, Wilson A, Mori TA . Effects of a lifestyle programme on ambulatory blood pressure and drug dosage in treated hypertensive patients: a randomized controlled trial. J Hypertens 2005; 23: 1241–1249.
Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER 3rd, Simons-Morton DG, Karanja N, Lin PH, DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001; 344: 3–10.
Fujita T . Mineralocorticoid receptors, salt-sensitive hypertension, and metabolic syndrome. Hypertension 2010; 55: 813–818.
Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK . Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ 2007; 334: 885–888.
The Hypertension Prevention Trial: three-year effects of dietary changes on blood pressure. Hypertension Prevention Trial Research Group. Arch Intern Med 1990; 150: 153–162.
Whelton PK, Kumanyika SK, Cook NR, Cutler JA, Borhani NO, Hennekens CH, Kuller LH, Langford H, Jones DW, Satterfield S, Lasser NL, Cohen JD . Efficacy of nonpharmacologic interventions in adults with high-normal blood pressure: results from phase 1 of the Trials of Hypertension Prevention. Trials of Hypertension Prevention Collaborative Research Group. Am J Clin Nutr 1997; 65 (2 Suppl): 652S–660S.
Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith West D, Milas NC, Mattfeldt-Beman M, Belden L, Bragg C, Millstone M, Raczynski J, Brewer A, Singh B, Cohen J, Trials for the Hypertension Prevention Research Group. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med 2001; 134: 1–11.
Kumanyika SK, Cook NR, Cutler JA, Belden L, Brewer A, Cohen JD, Hebert PR, Lasser VI, Raines J, Raczynski J, Shepek L, Diller L, Whelton PK, Yamamoto M, Trials of Hypertension Prevention Collaborative Research Group. Sodium reduction for hypertension prevention in overweight adults: further results from the Trials of Hypertension Prevention Phase II. J Hum Hypertens 2005; 19: 33–45.
Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar S, Lacy CR . Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Nonpharmacologic Interventions in the Elderly (TONE). Arch Intern Med 2001; 161: 685–693.
Thorogood A, Mottillo S, Shimony A, Filion KB, Joseph L, Genest J, Pilote L, Poirier P, Schiffrin EL, Eisenberg MJ . Isolated aerobic exercise and weight loss: a systematic review and meta-analysis of randomized controlled trials. Am J Med 2011; 124: 747–755.
Douketis JD, Macie C, Thabane L, Williamson DF . Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes (Lond) 2005; 29: 1153–1167.
Fagard RH . Exercise is good for your blood pressure: effects of endurance training and resistance training. Clin Exp Pharmacol Physiol 2006; 33: 853–856.
Cox KL, Puddey IB, Morton AR, Burke V, Beilin LJ, McAleer M . Exercise and weight control in sedentary overweight men: effects on clinic and ambulatory blood pressure. J Hypertens 1996; 14: 779–790.
Cornelissen VA, Fagard RH, Coeckelberghs E, Vanhees L . Impact of resistance training on blood pressure and other cardiovascular risk factors: a meta-analysis of randomized, controlled trials. Hypertension 2011; 58: 950–958.
Shaw K, Gennat H, O'Rourke P, Del Mar C . Exercise for overweight or obesity. Cochrane Database Syst Rev 2006; (4: CD003817.
Fogelholm M, Kukkonen-Harjula K . Does physical activity prevent weight gain—a systematic review. Obes Rev 2000; 1: 95–111.
Jakicic JM . The effect of physical activity on body weight. Obesity (Silver Spring) 2009; 17 (Suppl 3): S34–S38.
Proper KI, Singh AS, van Mechelen W, Chinapaw MJ . Sedentary behaviors and health outcomes among adults: a systematic review of prospective studies. Am J Prev Med 2011; 40: 174–182.
Whitman IR, Agarwal V, Nah G, Dukes JW, Vittinghoff E, Dewland TA, Marcus GM . Alcohol abuse and cardiac disease. J Am Coll Cardiol 2017; 69: 13–24.
Puddey IB, Beilin LJ . Alcohol is bad for blood pressure. Clin Exp Pharmacol Physiol 2006; 33: 847–852.
Sayon-Orea C, Martinez-Gonzalez MA, Bes-Rastrollo M . Alcohol consumption and body weight: a systematic review. Nutr Rev 2011; 69: 419–431.
Puddey IB, Parker M, Beilin LJ, Vandongen R, Masarei JR . Effects of alcohol and caloric restrictions on blood pressure and serum lipids in overweight men. Hypertension 1992; 20: 533–541.
Canoy D, Wareham N, Luben R, Welch A, Bingham S, Day N, Khaw KT . Cigarette smoking and fat distribution in 21,828 British men and women: a population-based study. Obes Res 2005; 13: 1466–1475.
Rhee MY, Na SH, Kim YK, Lee MM, Kim HY . Acute effects of cigarette smoking on arterial stiffness and blood pressure in male smokers with hypertension. Am J Hypertens 2007; 20: 637–641.
Simpson SA, Shaw C, McNamara R . What is the most effective way to maintain weight loss in adults? BMJ 2011; 343: d8042.
Van Dorsten B, Lindley EM . Cognitive and behavioral approaches in the treatment of obesity. Med Clin North Am 2011; 95: 971–988.
Greaves CJ, Sheppard KE, Abraham C, Hardeman W, Roden M, Evans PH, Schwarz P, IMAGE Study Group. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health 2011; 11: 119.
Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, Vollmer WM, Gullion CM, Funk K, Smith P, Samuel-Hodge C, Myers V, Lien LF, Laferriere D, Kennedy B, Jerome GJ, Heinith F, Harsha DW, Evans P, Erlinger TP, Dalcin AT, Coughlin J, Charleston J, Champagne CM, Bauck A, Ard JD, Aicher K, Weight Loss Maintenance Collaborative Research Group. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA 2008; 299: 1139–1148.
Funk KL, Stevens VJ, Appel LJ, Bauck A, Brantley PJ, Champagne CM, Coughlin J, Dalcin AT, Harvey-Berino J, Hollis JF, Jerome GJ, Kennedy BM, Lien LF, Myers VH, Samuel-Hodge C, Svetkey LP, Vollmer WM . Associations of internet website use with weight change in a long-term weight loss maintenance program. J Med Internet Res 2010; 12: e29.
Tham JC, le Roux CW, Docherty NG . Cardiovascular, renal and overall health outcomes after bariatric surgery. Curr Cardiol Rep 2015; 17: 34.
Colquitt JL, Pickett K, Loveman E, Frampton GK . Surgery for weight loss in adults. Cochrane Database Syst Rev 2014; 8: CD003641.
Petersen KS, Blanch N, Keogh JB, Clifton PM . Effect of weight loss on pulse wave velocity: systematic review and meta-analysis. Arterioscler Thromb Vasc Biol 2015; 35: 243–252.
Boido A, Ceriani V, Cetta F, Lombardi F, Pontiroli AE . Bariatric surgery and prevention of cardiovascular events and mortality in morbid obesity: mechanisms of action and choice of surgery. Nutr Metab Cardiovasc Dis 2015; 25: 437–443.
de Jonge L, Moreira EA, Martin CK, Ravussin E, Pennington CALERIE Team. Impact of 6-month caloric restriction on autonomic nervous system activity in healthy, overweight, individuals. Obesity (Silver Spring) 2010; 18: 414–416.
Jassil FC, Manning S, Lewis N, Steinmo S, Kingett H, Lough F, Pucci AB, Cheung WH, Finer N, Walker J, Doyle J, Batterham RL . Feasibility and impact of a combined supervised exercise and nutritional-behavioral intervention following bariatric surgery: a pilot study. J Obes 2015; 2015: 693829.
Kushner RF . Weight loss strategies for treatment of obesity. Prog Cardiovasc Dis 2014; 56: 465–472.
Coen PM, Goodpaster BH . A role for exercise after bariatric surgery? Diabetes Obes Metab 2015; 18: 16–23.
Arena R, Lavie CJ . The healthy lifestyle team is central to the success of accountable care organizations. Mayo Clin Proc 2015; 90: 572–576.
Butsch WS . Obesity medications: what does the future look like? Curr Opin Endocrinol Diabetes Obes 2015; 22: 360–366.
Lavie CJ, Milani RV, Ventura HO . Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol 2009; 53: 1925–1932.
Lavie CJ, Milani RV, O’Keefe JH . The Russert impact: a golden opportunity to promote primary coronary prevention. Ochsner J 2008; 8: 108–113.
Artham SM, Lavie CJ, Milani RV, Patel DA, Verma A, Ventura HO . Clinical impact of left ventricular hypertrophy and implications for regression. Prog Cardiovasc Dis 2009; 52: 153–167.
Lavie CJ, Patel DA, Milani RV, Ventura HO, Shah S, Gilliland Y . Impact of echocardiographic left ventricular geometry on clinical prognosis. Prog Cardiovasc Dis 2014; 57: 3–9.
DiNicolantonio JJ, Fares H, Niazi AK, Chatterjee S, D'Ascenzo F, Cerrato E, Biondi-Zoccai G, Lavie CJ, Bell DS, O'Keefe JH . β-Blockers in hypertension, diabetes, heart failure and acute myocardial infarction: a review of the literature. Open Heart 2015; 2: e000230.
Marcus JA, Pothineni A, Marcus CZ, Bisognano JD . The role of obesity and obstructive sleep apnea in the pathogenesis and treatment of resistant hypertension. Curr Hypertens Rep 2014; 16: 411.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Leggio, M., Lombardi, M., Caldarone, E. et al. The relationship between obesity and hypertension: an updated comprehensive overview on vicious twins. Hypertens Res 40, 947–963 (2017). https://doi.org/10.1038/hr.2017.75
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2017.75
Keywords
This article is cited by
-
Remnant cholesterol and risk of incident hypertension: a population-based prospective cohort study
Hypertension Research (2024)
-
Predictors of Cardiac Autonomic Dysfunction in Obesity-Related Hypertension
High Blood Pressure & Cardiovascular Prevention (2024)
-
Long-Term Protective Effects and Mechanisms of Gastric Bypass Surgery on the Kidneys in Hypertensive Obese Rat
Obesity Surgery (2024)
-
ANMCO (Italian Association of Hospital Cardiologists) scientific statement: obesity in adults—an approach for cardiologists
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2024)
-
Thymoquinone mitigates obesity and diabetic parameters through regulation of major adipokines, key lipid metabolizing enzymes and AMPK/p-AMPK in diet-induced obese rats
3 Biotech (2024)