Abstract
This study aimed to examine the relationship between the ratio of monocyte frequency to high-density lipoprotein (HDL) cholesterol level (the monocyte/HDL ratio (MHR)) and asymptomatic organ damage (AOD) in primary hypertension (PHT). A total of 366 participants were enrolled in the study, including 275 cases currently being followed up after a diagnosis of PHT in our clinic and 91 healthy volunteers. The MHR was higher in patients with PHT than in individuals in the control group. In the PHT group, the MHR was higher in patients with AOD (AOD+) than in patients without AOD (AOD−). In the correlation analyses performed in the PHT group, there were positive correlations between the MHR and the following AOD indicators: carotid intima media thickness, left ventricular mass index, urinary protein levels and urinary albumin levels. In a multivariate linear regression analysis, the MHR was found to be an independent risk factor associated with these indicators of AOD. In conclusion, our study shows that MHR is associated with AOD in patients with PHT.
Similar content being viewed by others
Introduction
Primary hypertension (PHT) has become a serious public health problem all across the world and is a serious condition that plays a role in the etiopathogenesis of cardiovascular and cerebrovascular diseases.1 High blood pressure caused by increased cardiac output and peripheral vascular resistance causes damage to cardiac and vascular tissues, and leads to asymptomatic atherosclerosis and further organ dysfunction.2, 3 Asymptomatic organ damage (AOD), which does not initially cause functional impairment in the related tissue, may cause irreversible organ dysfunction when it reaches a critical level.4, 5 Therefore, it is important to diagnose hypertension in the AOD stage, and start treatment to mitigate or prevent damage.
AOD in PHT is associated with the level of damage in the endothelial tissue in the cardiovascular system; this damage continues to be asymptomatic if it remains at a level that is insufficient to impair the functionality of the vascular structure.6 In clinical practice, this damage is indicated by an increased carotid intima media thickness (CIMT), an increased left ventricular mass index (LVMI), increased current-induced dilation in the radial artery and increased excretion of urinary proteins.7 However, each of these indicators requires advanced study and analysis by a specialist. Thus, recent studies have aimed to search for indicators and indexes for AOD prediction that are simple, economical and feasible in a clinical setting. The ability of monocyte frequency and related indexes to predict the presence of AOD has been investigated in many studies.
Monocyte frequency is known to increase in atherosclerosis and indicate chronic inflammation. Considering the pathogenesis of atherosclerosis, monocytes—among other inflammatory cells—are known to play an active role both in the generation of atheroma plaques at the vascular level and in endothelial dysfunction. Consistent with this, the association between increased monocyte frequency (secondary to chronic inflammation) and many cases of atherosclerosis has been demonstrated in several previous studies.8, 9, 10, 11, 12 As monocytes are only one of many types of inflammatory cell that play a role in inflammation during the pathogenesis of cardiovascular diseases, macrophage frequency is considered to be a weak predictor of atherosclerosis. For this reason, we sought to create an improved index that included a marker that diminishes in the presence of atherosclerosis, as well as monocyte frequency, which is known to increase in this setting. To this end, we created a ratio of monocyte frequency to high-density lipoprotein (HDL) cholesterol (the monocyte/HDL ratio (MHR)), given that HDL cholesterol is found at low levels in patients with atherosclerosis. The MHR has previously been demonstrated to be a useful marker for the identification of coronary artery disease, slow coronary flow and saphenous vein graft syndromes.8, 9, 10, 11
We hypothesized that the MHR, which has been shown to be a good predictor of atherosclerosis, would also be a good predictor of AOD in patients with PHT. Therefore, we aimed to examine the association between the MHR and AOD in patients with PHT.
Methods
This investigation involved a retrospective study that was carried out at Ankara Numune Training and Research Hospital, Internal Medicine Clinic, from August 2016 to November 2016.
Study population
The study comprised a total of 366 participants aged over 18 years, and included 272 individuals who had been diagnosed with PHT and were being monitored in our clinic, and 91 healthy volunteers. Subjects who visited our hospital for a check-up did not have a known chronic disease, and were not taking any medications, were included in the control group.
The following patients were excluded from the study: those who had secondary hypertension, diabetes mellitus, obesity, acute or chronic kidney disease, the presence of proteinuria at the nephritic level, documented coronary artery disease, cardiac failure, peripheral arterial disease, cerebrovascular disease, malignancy, liver diseases, rheumatic diseases, or active or chronic infection; those who were taking antioxidant substances or lipid-reducing medicines; and those who smoked, consumed alcohol or took vitamin tablets.
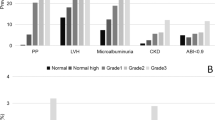
The following parameters were considered to be indicators of AOD: urinary albumin excretion >30 mg per day or proteinuria >150 mg per day, LVMI >95 g m−2 in females and >115 g m−2 in males, CIMT >0.9 mm or the presence of a plaque (or plaques) in the carotid arteries.13 Patients who lacked all of these indicators were classified as AOD-negative (AOD−); those who had one AOD indicator as AOD grade I; those who had two indicators as AOD grade II; those who had three indicators as AOD grade III; and those who had four indicators as AOD grade IV.
Body mass index (BMI) was calculated by dividing an individual’s body weight by the square of their height in meters (BMI=kg m−2).
The study was approved by the local ethics board.
Biochemical parameters
Fasting laboratory parameters were measured when patients were diagnosed with PHT upon their admission to hospital and values were recorded in the patients’ electronic medical records. For these laboratory parameters, the measurement tools and methods used in the biochemistry department of our hospital are described below.
The triglyceride and total cholesterol levels were measured using the enzymatic colorimetric method, HDL cholesterol was measured using the homogeneous enzymatic colorimetric method, and urinary protein levels at 24 h were measured using the microalbumin turbidimetric method and analyzed using a Hitachi Modular P800 autoanalyzer (Roche Diagnostics, Indianapolis, IN, USA). Low-density lipoprotein cholesterol was calculated using the Friedewald formula.14 Erythrocyte and thrombocyte frequencies were measured using the impedance (resistance) method, leukocyte frequency was quantified by optic laser scatter and other hemogram parameters were measured using a Sysmex XE 2100 hematology analyzer (Roche Diagnostic). Hemoglobin levels were measured photometrically.
Blood pressure measurement
After 5 min of rest after admission to hospital, participants were subjected to three different blood pressure measurements at 5-min intervals. The mean value of the three measurements was recorded as the final measurement. An Omron M3 automatic sphygmomanometer (Omron Healthcare, Tokyo, Japan) that was calibrated according to the European Society of Hypertension International Protocol 2002 and 201015 was used for blood pressure measurements.
Echocardiographic examination
Echocardiography was performed by a cardiologist who did not have knowledge of the study. A standard echocardiography machine (2.5 MHz transducer, Vivid 7; GE-Vingmed Ultrasound AS, Horten, Norway) was used for echocardiography measurements. Left-ventricle mass was calculated using two-dimensional echocardiographic measurements and the Devereux formula.
The Devereux formula was indexed to: LVM=1.04 × [(IVST+PWT+LVDd)3–(LVDd)3]–13.6 and body surface area. LVMI values of >95 g m−2 in females and >115 g m−2 in males were considered to be indicators of left-ventricle hypertrophy.
Carotid ultrasonography
CIMT was measured when the patients lay on their back and put two hands under their head. Measurements were performed by a radiologist who did not know about the clinical condition of patients using a high-resolution B-mode device (Logic 7; GE Med., Chicago, IL, USA). Measurements were taken from the right and left main carotid arteries using a linear probe and an automatic system. Measurements were taken at three locations: 2 cm away from the site at which the left carotid artery branches from the aorta, 2 cm away from the site at which the right carotid artery branches from the brachiocephalic trunk and at the bifurcation of the internal carotid arteries. Longitudinal measurements were made at the distance defined as the vein lumen echogenicity and media-adventitia echogenicity. Intima media thickness was measured by ultrasound as the distance between the sites at which echogenicity was detected for the vein lumen and for the media-adventitia border.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) for Windows 20 (IBM SPSS, Chicago, IL, USA). The extent to which the data followed a normal distribution was evaluated using the Kolmogorov–Smirnov test. Among numeric variables, those that followed a normal distribution are provided as means±s.d., and those that did not follow a normal distribution are provided as medians (along with the range). Categorical variables are provided as numbers and percentages. T-tests (for numeric variables that followed a normal distribution) and Mann–Whitney U-tests (for numeric variables that did not follow normal distribution) were used in independent samples for the identification of factors associated with two-category risk groups. ANOVA (for numeric variables that followed a normal distribution) and the Kruskal–Wallis H-test (for numeric variables that did not follow a normal distribution) were used in the identification of factors associated with three-category risk groups. The χ2 test and Fisher’s exact χ2 test were used to compare categorical data. The correlation between numeric variables was examined with Pearson and Spearman correlation analyses. Stepwise multivariate linear regression analysis was used to identify independent predictors of the indicators of atherosclerosis. Stepwise multivariate logistic regression analysis was used to predict AOD. Stepwise multivariate multinomial logistic regression analysis was used to predict the grades of AOD. Numeric prediction values generated by independent researchers were examined using receiver operating characteristic curve analysis according to the Youden index method. P<0.05 was considered to be significant in statistical analyses.
Results
The study population comprised 366 individuals, including 275 patients with PHT and 91 healthy controls. The gender ratio, mean age and mean BMI did not differ between the PHT group and the control group (P>0.05). The mean monocyte frequency in the PHT group was higher than that in the control group (306.4±111 vs. 566.9±166.2 μl; P<0.001). Considering the lipid levels, the mean HDL cholesterol levels were lower (67.9±58.8 vs. 47.7±11.7 mg dl−1; P<0.001) and the median triglyceride levels were higher (113 vs. 142 mg dl−1; P<0.001) in the PHT group than in the control group. The mean MHR was higher in the PHT group than in the control group (4.9±1.7 vs. 12.6±5.4; P<0.001). The median urinary protein levels, median urinary albumin level, mean LVMI and mean CIMT values were higher in the PHT group than in the control group.
In patients with PHT, the urinary protein levels positively correlated with the monocyte frequency (r=0.240; P=0.020) and with the MHR (r=0.327; P<0.001), and negatively correlated with the HDL cholesterol levels (r=−0.255; P=0.010). In addition, the urinary albumin levels positively correlated with the monocyte frequency (r=0.317; P=0.001) and with the MHR (r=0.482; P<0.001), and negatively correlated with the HDL cholesterol levels (r=−0.306; P=0.001). In patients with PHT, the LVMI positively correlated with age (r=0.220; P=0.046), with the monocyte frequency (r=0.379; P<0.001), and with the MHR (r=0.396; P<0.001), and negatively correlated with the HDL cholesterol levels (r=−0.285; P=0.002). The CIMT values positively correlated with age (r=0.252; P<0.001), with monocyte frequency (r=0.339; P<0.001), and with the MHR (r=0.486; P<0.001), and negatively correlated with the HDL cholesterol levels (r=−0.390; P<0.001) (Table 1).
Independent predictors of AOD
Variables related to AOD are provided in Table 1. Accordingly, these variables were included in a multivariate linear regression analysis. As urinary protein and urinary albumin levels did not demonstrate normal distributions, these data were converted into normal distributions by logarithmic conversion.
The log(urinary albumin) (non-standardized regression number±s.e. (B±s.e.)=0.329±0.026; P<0.001) and the MHR (B±s.e.=0.110±0.030; P<0.001) were found to be independent predictors of the log(urinary protein). The log(urinary protein) (B±s.e.=1.131±0.089; P<0.001) and the MHR (B±s.e.=0.430±0.050; P<0.001) were found to be independent predictors of the log(urinary albumin). The CIMT values (B±s.e.=2.524±0.663; P<0.001) and the MHR (B±s.e.=1.576±0.267; P<0.001) were found to be independent predictors of the LVMI. The LVMI (B±s.e.=0.200±0.052; P<0.001) and the MHR (B±s.e.=1.814±0.197; P<0.001) were found to be independent predictors of the CIMT (Table 2).
Demographic and laboratory findings in the presence and absence of AOD
AOD was identified in 75.6% of patients with PHT. Demographic and laboratory findings for the control group, and the AOD− and AOD+ groups are provided in Table 3. In AOD+ patients, the gender ratio was similar to that of both the control group and the AOD− group; similarly, the BMI, systolic blood pressure and diastolic blood pressure did not differ among groups. The mean age was higher in the AOD+ group than in the AOD− and control groups (control: 50.2±11.5 years vs. AOD−: 48.8±10.2 years vs. AOD+: 53.7±12.3 years; P=0.003).
In the AOD+ group, the mean monocyte frequency was higher and the mean HDL cholesterol levels were lower than in the other groups. The mean MHR was higher in the AOD+ group than in the other groups (4.9±1.7 vs. 8.1±0.5 vs. 14.1±5.4; P<0.001) (Table 3).
When individuals in the PHT group were classified according to the severity of AOD, the mean monocyte frequency and the MHR increased, and the mean HDL cholesterol level decreased, as the severity of AOD severity increased from grade I to IV (Table 4).
Independent predictors of the presence and grade of AOD
When AOD was graded according to the number of organs involved (grade I–IV), a stepwise multivariate logistic regression analysis showed that the MHR was an independent predictor of all grades of AOD (Table 5).
An MHR value higher than 9.06 with 94.2% sensitivity and 100% specificity was found to be a predictor of AOD (area under the curve±s.e.=0.993±0.002; P<0.001). According to the area under the curve, the MHR had the highest diagnostic discrimination relative to the other AOD indicators in the prediction of the presence of AOD (Figure 1a). The diagnostic discrimination of the MHR in predicting the AOD grade is shown in Figure 1b.
Discussion
This study is the first to report that the MHR is higher in patients with PHT than in healthy controls. In the PHT group, the MHR was higher in AOD+ patients than in AOD− patients. When the AOD severity was classified according to the number of organs involved (grade I–IV), the MHR was found to increase with increasing AOD severity. In the correlation analyses of the PHT group, positive correlations were found between the MHR and the following AOD indicators: CIMT, LVMI, urinary protein levels and urinary albumin levels. In the multivariate linear regression analysis, the MHR was found to be an independent risk factor for increased CIMT, LVMI, urinary protein levels and urinary albumin levels.
Endothelial dysfunction is identified through the measurement of indirect indicators in clinical practice and in most studies in the literature.16 These indicators include CIMT, which is measured by carotid doppler ultrasonography;17 LVMI, which can be evaluated by echocardiography; current-induced dilation, which can be measured from the radial artery; and protein and albumin excretion from the kidney.18 However, each of these indicators requires advanced study and analysis by a specialist. Therefore, researchers have begun to search for methods and applications that could be used in clinical practice to examine endothelial dysfunction and that are economical and easy to perform by everyone at any place. While some studies have examined the importance of some indicators as markers of endothelial dysfunction, others have examined certain laboratory parameters and the importance of indexes generated by the proportioning of these parameters. We examined the importance of the MHR, which is calculated by proportioning two different laboratory parameters that have a close relationship to endothelial dysfunction. The HDL cholesterol level is a lipid parameter that decreases in the presence of endothelial dysfunction and atherosclerosis, and monocyte frequency is a hematological parameter that increases during inflammation. Therefore, theoretically, HDL cholesterol levels decrease while monocyte frequency increases in cases of endothelial dysfunction; thus, the MHR value increases.
Many recent studies have assessed the MHR. Most of these studies focused on the diagnosis, prognosis and severity of acute coronary syndrome.9, 19, 20, 21, 22, 23 These studies demonstrated that the MHR was closely related to atherosclerosis and ischemic findings. In addition, an increase in the MHR was correlated with the increased severity of ischemia and atherosclerosis. Therefore, many authors have suggested that the MHR could be a good indicator of disease in patients admitted to hospital with acute coronary syndrome.
Other studies have examined the role of the MHR in chronic kidney failure and contrast nephropathy.8, 24 An increased MHR was shown to be associated with a reduced glomerular filtration rate in patients with chronic kidney failure and the MHR was found to be a predictor of poor cardiovascular outcomes in patients with chronic kidney failure. The MHR was also found to be a good indicator of whether contrast nephropathy will develop after percutaneous coronary intervention in patients with ST-elevation myocardial infarction.
Furthermore, the MHR has been highlighted as a good predictor of whether atrial fibrillation will recur after interventional surgery in patients with atrial fibrillation.25
In our literature review, we did not encounter any study that examined the role of the MHR in patients with PHT. However, it is not known how this index varies in cases of subclinical atherosclerosis induced by PHT. The variability of this index after the development of cardiovascular disease is indeed an expected finding because, as mentioned previously, atherosclerosis is a chronic inflammatory process. In atherosclerosis, monocyte frequency is expected to increase as a result of the secretion of inflammatory mediators, and HDL cholesterol levels are known to decrease in atherosclerosis.
Consistent with the fact that PHT is an atherosclerotic and chronic inflammatory condition, we observed a higher MHR in patients with PHT than in control individuals. In the PHT group, the MHR was higher in AOD+ patients than in AOD− patients. We found that the MHR increased as the AOD grade increased. Our data reveal a decrease in HDL cholesterol levels and a concomitant increase in monocyte frequency in patients with subclinical atherosclerosis. We conclude that the pathophysiological event in the pathogenesis of this condition is directly related to endothelial dysfunction, which occurs as a result of mechanical or shear stress in PHT, because the level of inflammation increases in this setting, and lipid parameters fluctuate as atherosclerosis is triggered.5, 26 The findings that the studied indicators of AOD are correlated with the MHR and that the MHR is an independent predictor of AOD in our regression analysis suggest that the MHR can be used as an indicator of AOD that develops as a result of PHT.
The biggest limitation of this study is the cross-sectional design. Another limitation is that we did not study high-sensitivity inflammatory indicators to demonstrate chronic inflammation.
In conclusion, our study found that the MHR is an independent predictor of AOD that occurs secondary to PHT. For this index to be used as an indicator of AOD in clinical practice, the MHR needs to be evaluated after the application of preventive and mitigating treatments in patients with AOD. If the findings of such studies are consistent with those of this study, the MHR may be a good indicator for the diagnosis and monitoring of AOD; however, this requires further research.
References
Schiffrin EL, Campbell NR, Feldman RD, Kaczorowski J, Lewanczuk R, Padwal R, Tobe SW . Hypertension in Canada: past, present, and future. Ann Glob Health 2016; 82: 288–299.
Lackland DT . Controlling hypertension to prevent target organ damage: perspectives from the World Hypertension League President. Ethn Dis 2016; 26: 267–270.
Ojji DB, Libhaber E, Atherton JJ, Abdullahi B, Nwankwo A, Sliwa K . Risk-factor profile and comorbidities in 2398 patients with newly diagnosed hypertension from the Abuja Heart Study. Medicine (Baltimore) 2015; 94: e1660.
Mir S, Sozeri B, Deveci M, Ozdemir K, Gun ZH, Dincel N, Gozuoglu G, Yilmaz E, Kaplan Bulut I . Cardiovascular functional and structural changes in children with primary hypertension. Minerva Pediatr 2016; 68: 27–35.
Ates I, Ozkayar N, Topcuoglu C, Dede F . Relationship between oxidative stress parameters and asymptomatic organ damage in hypertensive patients without diabetes mellitus. Scand Cardiovasc J 2015; 49: 249–256.
Ates I, Ozkayar N, Ates H, Karakulak UN, Kursun O, Topcuoglu C, Inan B, Yilmaz N . Elevated circulating sST2 associated with subclinical atherosclerosis in newly diagnosed primary hypertension. Hypertens Res 2016; 39: 513–518.
Ates I, Ozkayar N, Akyel F, Topcuoglu C, Akyel S, Barca AN, Dede F . The relationship between asymptomatic organ damage, and serum soluble tumor necrosis factor-like weak inducer of apoptosis (sTWEAK) and Interleukin-17 A (IL-17 A) levels in non-diabetic hypertensive patients. BMC Nephrol 2014; 15: 159.
Sag S, Yildiz A, Aydin Kaderli A, Gul BC, Bedir O, Cegilli E, Ozdemir B, Can FE, Aydinlar A . Association of monocyte to HDL cholesterol level with contrast induced nephropathy in STEMI patients treated with primary PCI. Clin Chem Lab Med 2016; 55: 132–138.
Cetin MS, Ozcan Cetin EH, Kalender E, Aydin S, Topaloglu S, Kisacik HL, Temizhan A . Monocyte to HDL cholesterol ratio predicts coronary artery disease severity and future major cardiovascular adverse events in acute coronary syndrome. Heart Lung Circ 2016; 25: 1077–1086.
Canpolat U, Cetin EH, Cetin S, Aydin S, Akboga MK, Yayla C, Turak O, Aras D, Aydogdu S . Association of monocyte-to-HDL cholesterol ratio with slow coronary flow is linked to systemic inflammation. Clin Appl Thromb Hemost 2016; 22: 476–482.
Akboga MK, Canpolat U, Sahinarslan A, Alsancak Y, Nurkoc S, Aras D, Aydogdu S, Abaci A . Association of serum total bilirubin level with severity of coronary atherosclerosis is linked to systemic inflammation. Atherosclerosis 2015; 240: 110–114.
Gang L, Yanyan Z . Increased neutrophil to lymphocyte ratio in persons suffering from hypertension with hyperhomocysteinemia. Hypertens Res 2016; 39: 606–611.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de Borne P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De Buyzere M, De Geest S, Derumeaux GA, Erdine S, Farsang C, Funck-Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Ryden L, Sirenko Y, Stanton A, Struijker-Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M, Wood DA . 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34: 2159–2219.
Friedewald WT, Levy RI, Fredrickson DS . Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502.
O'Brien E, Atkins N, Stergiou G, Karpettas N, Parati G, Asmar R, Imai Y, Wang J, Mengden T, Shennan A . Working Group on Blood Pressure Monitoring of the European Society of H. European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit 2010; 15: 23–38.
Thompson JE, Smith W, Ware LJ, MCM C, van Rooyen JM, Huisman HW, Malan L, Malan NT, Lammertyn L, Schutte AE . Masked hypertension and its associated cardiovascular risk in young individuals: the African-PREDICT study. Hypertens Res 2016; 39: 158–165.
Hirata C, Miyai N, Idoue A, Utsumi M, Hattori S, Iwahara A, Uematsu Y, Shiba M, Arita M . Effect of metabolic syndrome components and their clustering on carotid atherosclerosis in a sample of the general Japanese population. Hypertens Res 2016; 39: 362–366.
Ates I, Bulut M, Ozkayar N, Dede F . Association between high platelet indices and proteinuria in patients with hypertension. Ann Lab Med 2015; 35: 630–634.
Arisoy A, Altunkas F, Karaman K, Karayakali M, Celik A, Ceyhan K, Zorlu C . Association of the monocyte to HDL cholesterol ratio with thrombus burden in patients with ST-segment elevation myocardial infarction. Clin Appl Thromb Hemost (e-pub ahead of print 16 August 2016; doi: 10.1177/1076029616663850).
Tok D, Turak O, Yayla C, Ozcan F, Tok D, Cagli K . Monocyte to HDL ratio in prediction of BMS restenosis in subjects with stable and unstable angina pectoris. Biomark Med 2016; 10: 853–860.
Akboga MK, Balci KG, Maden O, Ertem AG, Kirbas O, Yayla C, Acar B, Aras D, Kisacik H, Aydogdu S . Usefulness of monocyte to HDL-cholesterol ratio to predict high SYNTAX score in patients with stable coronary artery disease. Biomark Med 2016; 10: 375–383.
Kundi H, Kiziltunc E, Cetin M, Cicekcioglu H, Cetin ZG, Cicek G, Ornek E . Association of monocyte/HDL-C ratio with SYNTAX scores in patients with stable coronary artery disease. Herz 2016; 41: 523–529.
Cetin EH, Cetin MS, Canpolat U, Aydin S, Topaloglu S, Aras D, Aydogdu S . Monocyte/HDL-cholesterol ratio predicts the definite stent thrombosis after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Biomark Med 2015; 9: 967–977.
Kanbay M, Solak Y, Unal HU, Kurt YG, Gok M, Cetinkaya H, Karaman M, Oguz Y, Eyileten T, Vural A, Covic A, Goldsmith D, Turak O, Yilmaz MI . Monocyte count/HDL cholesterol ratio and cardiovascular events in patients with chronic kidney disease. Int Urol Nephrol 2014; 46: 1619–1625.
Canpolat U, Aytemir K, Yorgun H, Sahiner L, Kaya EB, Cay S, Topaloglu S, Aras D, Oto A . The role of preprocedural monocyte-to-high-density lipoprotein ratio in prediction of atrial fibrillation recurrence after cryoballoon-based catheter ablation. Europace 2015; 17: 1807–1815.
Ates I, Ozkayar N, Inan B, Yilmaz FM, Topcuoglu C, Neselioglu S, Erel O, Dede F, Yilmaz N . Dynamic thiol/disulphide homeostasis in patients with newly diagnosed primary hypertension. J Am Soc Hypertens 2016; 10: 159–166.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Aydin, E., Ates, I., Fettah Arikan, M. et al. The ratio of monocyte frequency to HDL cholesterol level as a predictor of asymptomatic organ damage in patients with primary hypertension. Hypertens Res 40, 758–764 (2017). https://doi.org/10.1038/hr.2017.36
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2017.36
Keywords
This article is cited by
-
Association of carotid atherosclerotic plaque and intima-media thickness with the monocyte to high-density lipoprotein cholesterol ratio among low-income residents of rural China: a population-based cross-sectional study
BMC Public Health (2023)
-
Predictive value of monocyte to HDL-C ratio for coronary artery lesions and intravenous immunoglobulin resistance in Kawasaki disease
European Journal of Pediatrics (2023)
-
Monocyte to HDL ratio: a novel marker of resistant hypertension in CKD patients
International Urology and Nephrology (2022)
-
Monocyte to HDL cholesterol ratio as a marker of the presence and severity of obstructive sleep apnea in hypertensive patients
Scientific Reports (2021)
-
A risk biomarker for obstructive sleep apnea in patients with coronary artery disease: monocyte to high-density lipoprotein ratio
Sleep and Breathing (2021)