Abstract
Preeclampsia is a systemic vascular disorder characterized by new-onset hypertension and proteinuria after 20 weeks of gestation. This condition targets several organs, including the kidneys, liver and brain, and is the leading cause of maternal and perinatal morbidity and mortality. Furthermore, recent evidence has revealed preeclampsia as a significant risk factor for future cardiovascular diseases in these women. Over the past decade, increasing evidence has indicated that maternal angiogenic imbalances caused by placental antiangiogenic factors play a central role in the systemic vascular dysfunction underling preeclampsia. The severity of the maternal antiangiogenic state correlates closely with maternal and perinatal outcomes. Assessing angiogenic imbalance and several vascular function tests have also emerged as a way of detecting systemic vascular dysfunction during pregnancy. This review summarizes the current understanding of the pathophysiology of preeclampsia, its clinical applications and clinical evidence for future cardiovascular risks.
Similar content being viewed by others
Introduction
Preeclampsia, characterized by hypertension and proteinuria developing after 20 weeks of gestation, is a pregnancy-specific systemic vascular disorder. It is often accompanied by life-threatening events including seizures (eclampsia), renal failure, HELLP (hemolysis, elevated liver enzymes and low platelets) syndrome, cerebral hemorrhage, pulmonary edema and placental abruption. Adverse perinatal complications of preeclampsia include preterm delivery, fetal growth restriction and perinatal death. This condition affects 3 to 5% of pregnancies and causes substantial maternal and neonatal morbidity and mortality.1, 2 Although the clinical symptoms of preeclampsia resolve completely by 12 weeks postpartum, recent evidence has demonstrated an association between a history of preeclampsia and future cardiovascular events.3, 4, 5
Generalized maternal endothelial dysfunction caused by ‘placental factors’ has long been proposed as integral to systemic vascular dysfunction in preeclampsia, as delivering the placenta usually resolves this condition.6, 7, 8, 9 Over the past decade, a maternal antiangiogenic state caused by placental antiangiogenic factors has emerged as one of the most important mechanisms underlying this generalized endothelial dysfunction.10, 11, 12, 13 Increased placental soluble fms-like tyrosine kinase 1 (sFlt1) has been shown to mediate this maternal antiangiogenic state by antagonizing vascular endothelial growth factor (VEGF) and placental growth factor (PlGF).10
In clinical practice, hypertension severity does not necessarily correlate with maternal or perinatal adverse outcomes. A significant portion of pregnant women without hypertension develop severe complications such as eclampsia, pulmonary edema or HELLP syndrome.14, 15, 16 Conversely, it is not unusual for women who meet the conventional diagnostic criteria for preeclampsia to have no adverse complications. Furthermore, termination of the pregnancy, which often requires premature delivery, remains the only way to ameliorate the symptoms of preeclampsia.1, 2 Therefore, discovering additional, highly sensitive indicators to identify pregnant women at risk for adverse outcomes has been a matter of great interest.17, 18
Recently, the value of assessing the severity of the maternal antiangiogenic state has been evaluated as a method to identify pregnant women at risk for adverse outcomes. Several vascular function tests were also shown to be sensitive for detecting systemic vascular dysfunction during pregnancy. In addition, several therapeutic interventions designed to reverse maternal antiangiogenic states have been proposed. In this review, we summarize the current understanding of the pathophysiology of preeclampsia, its clinical applications and clinical evidence for future cardiovascular risks.
Angiogenic imbalance in preeclampsia
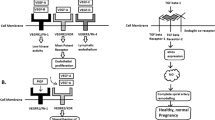
‘The two-stage theory’ (Figure 1) is widely accepted with regard to mechanisms of preeclampsia.19 In early placentation, trophoblast cells invade the wall of the maternal uterine spiral arteries and transform these arteries into large diameter vessels with low resistance to blood flow.20, 21 In preeclampsia, this transformation is impaired (abnormal placentation)22, 23, 24, 25 and the resulting hypoperfused placenta releases ‘placental factors’ into the maternal circulation that cause generalized endothelial dysfunction, leading to systemic vascular dysfunction. The primary cause of abnormal placentation remains unclear, but genetic, immunological and environmental factors are all likely to be involved.26
The two-stage theory of preeclampsia. A defective cytotrophoblast invasion into maternal uterine spiral arteries (abnormal placentation) has been proposed as an initial step (stage 1). The resulting hypoperfused placenta releases antiangiogenic factors (soluble fms-like tyrosine kinase 1 (sFlt1)) into the maternal circulation that induce maternal generalized endothelial dysfunction and systemic vascular dysfunction (stage 2). A full color version of this figure is available at the Hypertension Research journal online.
The identification of these ‘placental factors’ has been a primary research interest for decades. In 2003, three research groups (Maynard et al.,10 Koga et al.27 and Tsatsaris et al.28) reported abnormal increases in serum sFlt1 (also referred to soluble vascular endothelial growth factor receptor 1 (sVEGFR1)) in women with preeclampsia. Maynard et al.10 also reported that adenoviral gene transfer of sFlt1 to pregnant rats induced preeclamptic phenotypes, such as hypertension and proteinuria. Subsequently, Levine et al.11 showed that sFlt1 begins to increase before the onset of clinical preeclampsia symptoms, and that the level of sFlt1 correlates well with preeclampsia severity. These reports generated great enthusiasm for sFlt1 as the most promising ‘placental factor’, and angiogenic imbalance emerged as one of the most important pathogenic mechanisms of preeclampsia.
sFlt1 is an alternatively spliced version of VEGFR1 (Flt1) that retains the extracellular ligand-binding domain of VEGFR1 but lacks the transmembrane and cytoplasmic domains.29 VEGF interacts with VEGFR1 (Flt1) and VEGFR2 (KDR/FlK1), and PlGF specifically interacts with VEGFR1 (Flt1).30 sFlt1 is mainly released from the placenta into the maternal circulation,31 where it antagonizes VEGF and PlGF by binding to them and preventing their interaction with their endothelial receptors.29 As VEGF and PlGF are potent endothelial cell mitogens that regulate blood vessel development and homeostasis,32, 33, 34, 35 binding of VEGF and PlGF to sFlt1 results in an angiogenic imbalance that leads to generalized endothelial dysfunction and systemic vascular dysfunction (Figure 2). We exploited the model of preeclampsia in mice by specifically overexpressing sFlt1 in placenta.36 Consequently, the placenta produced excessive sFlt1 as gestation progressed, and the mice developed hypertension and proteinuria that resolved soon after delivery. The clinical observation that a VEGF inhibitor used as an anticancer treatment causes preeclampsia-like symptoms such as hypertension and proteinuria also supports the hypothesis that inducing an antiangiogenic state by inhibiting VEGF signaling contributes to the pathogenesis of preeclampsia.37, 38
Excessive soluble fms-like tyrosine kinase 1 (sFlt1) in women with preeclampsia. Vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) bind to VEGF receptor 1 (VEGFR1: also known as Flt1) and induce angiogenesis that is essential for normal pregnancy (a). In preeclampsia, the hypoperfused placenta produces excess soluble form of VEGFR1 (sFlt1) that antagonizes VEGF and PlGF in maternal serum and impairs angiogenesis (b). A full color version of this figure is available at the Hypertension Research journal online.
The mechanisms regulating sFlt1 production by trophoblastic cells are largely unknown. Placental hypoxia stimulates sFlt1 production in primary cytotrophoblast culture.39 Recently, the upregulation of endometrial VEGF was identified as an upstream regulator of sFlt1 production by trophoblastic cells.40 In addition, pleiotropic sFlt1 functions have recently attracted attention.41 Our group reported cytotoxic effects of sFlt1 against ovarian and colorectal cancer cell lines, and demonstrated therapeutic effects of sFlt1 in a mouse model of ovarian cancer.42
A placenta-derived soluble TGF-β coreceptor, endoglin (sEng), also induces a preeclampsia-like syndrome in concert with sFlt1 in pregnant rats.12 Serum sEng levels were significantly higher in women with preeclampsia, with this increase beginning before the onset of clinical symptoms.13, 43, 44 Thus, sFlt1 and sEng are thought to synergistically induce an antiangiogenic state.
Vascular pathophysiology of preeclampsia
Hypertension and proteinuria are hallmarks for the conventional diagnosis of preeclampsia. Normally during pregnancy, maternal circulation undergoes remarkable physiological adaptations, including increased intravascular volume and markedly decreased vascular resistance.45, 46, 47, 48 These adaptations result in an arterial blood pressure slightly below the nonpregnant level49, 50 and an enhanced glomerular filtration rate.51 In preeclampsia, increased placental sFlt1 is thought to increase peripheral vascular resistance (and thus arterial pressure) by counteracting VEGF- and PlGF-induced microvascular relaxation.10 VEGF signaling is also important for the maintenance of fenestrated endothelium; inhibiting VEGF signaling narrows the glomerular endothelial fenestrae and causes endothelial swelling, termed glomerular capillary endotheliosis, that decreases glomerular filtration rate and increases urinary protein excretion.52, 53 Inhibition of VEGF signaling also induces podocyte dysfunction that may contribute to glomerular capillary endotheliosis and renal thrombotic microangiopathy.54, 55
As described above, preeclampsia can be characterized as a systemic vascular disorder with generalized endothelial dysfunction caused by a maternal antiangiogenic state. Recently, several noninvasive vascular function tests have received considerable attention in nonpregnant subjects as they were found to relate more closely with endothelial function and future cardiovascular disease risk (CVD) than conventional brachial blood pressure measurements.56 Among these tests, flow-mediated dilation (FMD, a measure of conduit artery endothelial function), pulse wave analysis (a composite measure of conduit and resistance artery stiffness) and pulse wave velocity (PWV, a measure of conduit artery stiffness) have been increasingly incorporated into clinical practice in nonpregnant subjects.56 As these vascular function tests reflect endothelial function to varying degrees,57 they are expected to reflect the severity of systemic vascular dysfunction in women with preeclampsia to a varying degree.
FMD is a well-established technique for assessing endothelial dysfunction in a large vessel, the brachial artery, as well as predicting future cardiovascular risk.58, 59 Pregnancy is normally associated with increased FMD, reflecting enhanced endothelial function.60 A recent systematic review and meta-analysis reported significantly lower FMD in women with preeclampsia both before and after the onset of preeclampsia as well as for 3 years postpartum; these results indicate that endothelial dysfunction precedes the onset of preeclampsia and persists after pregnancy.61 These results also suggest the potential of FMD to predict preeclampsia, and represent a possible mechanism for future cardiovascular risk in these women.61
Pulse wave analysis measures the vascular compliance of the entire arterial tree62, 63, 64 and reflects relatively early changes in arterial aging.65 The pulse wave analysis indices of augmentation index (AIx) and central systolic pressure (CSP) are more closely correlated than conventional brachial blood pressure measurements with future cardiovascular events.66, 67, 68, 69 AIx and CSP decline markedly in normal pregnancy, reaching a nadir in mid-pregnancy and then increasing toward term.70, 71, 72 In women with preeclampsia, AIx and CSP are significantly elevated, suggesting increased systemic arterial compliance.73, 74 Abnormal AIx or CSP values have been observed from the first trimester of pregnancy in women who ultimately develop preeclampsia.75, 76 In addition, abnormal AIx or CSP might be involved in the pathogenesis of intrauterine growth restriction, a major phenotype in pregnancies with preeclampsia.77, 78 Magnesium sulfate decreases AIx in women with preeclampsia, suggesting a possible mechanism for seizure prophylaxis.79 Increased AIx even 6 to 24 months postpartum in women with a history of early-onset preeclampsia and intrauterine growth restriction may represent a possible mechanism for future cardiovascular risks in these women.80
PWV reflects histopathological changes in the structure and stiffness of conduit arteries that are relatively late changes in the process of arterial aging in large vessels.65, 81 PWV has also been reported to predict future cardiovascular events.82, 83 Brachial-to-ankle PWV decreases significantly in the second trimester of normal pregnancy.84 Several studies have suggested increased PWV in women with preeclampsia.71, 84 The increased PWV values have also been observed from the first trimester of pregnancy in women who later develop preeclampsia.85
Although further research is needed, these vascular function tests combined with assessing antiangiogenic imbalance could better provide a comprehensive picture of the vascular pathophysiology of preeclampsia.
Clinical implications of angiogenic imbalance
As previously mentioned, angiogenic imbalance is an important pathogenic mechanism of preeclampsia. In addition, this imbalance precedes the definitive diagnosis of preeclampsia.11, 13, 86, 87 Thus, assessing and, if possible, correcting this angiogenic imbalance may play important roles in various clinical settings, such as predicting, diagnosing, managing and treating preeclampsia.
Several studies have attempted to demonstrate the potential of assessing angiogenic imbalance as a predictor of preeclampsia.43, 87, 88, 89 In the first trimester of pregnancy, the measurement sensitivity for these factors is insufficient to predict the development of preeclampsia.89, 90, 91, 92 However, late in pregnancy, the level of angiogenic imbalance adequately predicts the development of preeclampsia.93, 94 In clinical settings, predicting adverse maternal and fetal outcomes is clearly more important than predicting the development of preeclampsia. In patients with ‘suspected’ preeclampsia, the severity of angiogenic imbalance correlated more closely than brachial blood pressure with maternal and neonatal complications, suggesting that assessing the severity of angiogenic imbalance may overcome the shortcomings of the conventional diagnostic criteria for preeclampsia.88, 94 For example, in women with suspected preeclampsia presenting at <34 weeks, 86.0% of women with an sFlt1/PlGF ratio of ⩾85 had iatrogenic delivery within 2 weeks compared with 15.8% of women with an sFlt1/PlGF ratio of <85,88 suggesting that women with preeclampsia and relatively low sFlt1/PlGF ratios could be managed expectantly.95 In addition to distinguishing pregnant women with and without preeclampsia,96, 97 assessing angiogenic imbalance can successfully differentiate preeclampsia from other diseases mimicking preeclampsia, such as chronic and gestational hypertension,98 acute and chronic glomerulonephritis,99 lupus nephritis100, 101 and non-HELLP syndrome-related thrombocytopenia.102 Assessing angiogenic imbalance for risk stratification as well as differential diagnoses for preeclampsia is expected to decrease the incidence of unnecessary iatrogenic preterm deliveries in women at low risk for adverse outcomes and to reduce resource utilization without increasing the risk of adverse maternal and neonatal outcomes.
Despite promising basic research investigating the therapeutic potential of reducing sFlt1 levels or raising PlGF levels,36, 103, 104, 105, 106 there is little clinical evidence that modulating angiogenic factors improves maternal or perinatal outcomes in women with preeclmapsia. However, in the only report in women with preeclampsia, extracorporeal removal of sFlt1 by dextran sulfate cellulose apheresis resulted in prolongation of pregnancy.107 Statins (HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A) reductase inhibitors, a class of lipid-lowering medications) promote PlGF expression in animal models,36 and a pilot human trial to support the safety and efficacy of a statin (pravastatin) for the prevention of preeclampsia in high-risk pregnant women was recently reported.108
Future cardiovascular risk of preeclampsia
Traditionally, preeclampsia was thought to completely resolve after delivery in otherwise healthy young women, but recent studies demonstrate that women with a history of preeclampsia have an increased risk of developing CVD later in life.3, 4, 5 In a large cohort study of 1 30 000 births, Smith et al.109 reported that women with preeclampsia had twice the risk of hospital admission and death because of ischemic heart disease over 15–19 years of follow-up compared with women without preeclampsia. According to a meta-analysis on the postpartum risk of CVD, the relative 10–15-year risk in women with a history of preeclampsia is 3.7 (95% confidence interval: 2.70−5.05) for hypertension, 2.16 (1.86−2.52) for ischemic heart disease, 1.81 (1.45−2.27) for cerebral infarction and 1.79 (1.37−2.33) for venous thrombosis.110
The precise mechanisms linking preeclampsia with future CVD may be complex, as both have common preexisting risk factors and endothelial dysfunction is considered a basic pathological feature underlying both diseases. As mentioned above, the systemic vascular dysfunction that occurs during preeclampsia persists after delivery61, 80 and may increase the risk of subsequent CVD. sFlt1 levels are increased in individuals with acute myocardial infarction and may predict the progression to heart failure in these patients.111 Depressed cardiac function in women with preeclampsia correlated with circulating sFlt1 levels, and exogenous sFlt1 induced cardiac dysfunction in a mouse model of peripartum cardiomyopathy.112 In women with a history of preeclampsia, increased sFlt1 and high-sensitivity C-reactive protein persisted from delivery to 5–8 years postpartum.113 Increased sFlt1 levels and sFlt1/PlGF ratio were also reported 1 year postpartum, and these parameters significantly correlated with the intima and media thickness of the common carotid artery as well as their ratio that indicates arterial aging.114
The occurrence of CVD later in life in women with a history of preeclampsia has also been reported in Japan,115, 116, 117 where the incidence of CVD is lower than in Western countries. In an analysis of Japanese women aged ⩾40 years using the ‘Boshi kenkou techou (Mother and baby health handbook)’, which includes information on blood pressure, proteinuria and maternal weight gain throughout the pregnancy, women with a history of preeclampsia had increased risk of antihypertensive medication use (odds ratio: 4.28 (2.14−8.57)) and dyslipidemic medication use (odds ratio: 3.20 (1.42−7.22)) compared with women without preeclampsia.118
Although there is an association between preeclampsia and CVD later in life, it is unclear whether early identification of women at high risk of CVD and subsequent intervention reduces the increased cardiovascular risk. Lifestyle interventions, including smoking cessation and weight reduction, has been reported to decrease cardiovascular risk after preeclampsia by 4−13%.119 In 2011, the American Heart Association guidelines advised yearly blood pressure, lipid profile and blood glucose concentration checks for women with a previous history of preeclampsia.120 In Japan, a lifestyle intervention is now recommended after delivery for women with preeclampsia,121 and the Japanese Society of Hypertension guidelines recommend referring to the ‘Boshi kenkou techou’ for health management in hypertensive women.122
Conclusion
Considerable advancement in the understanding of the pathophysiology of preeclampsia has occurred in the past decade. A maternal antiangiogenic state induced by placental antiangiogenic factors has emerged as one of the most important mechanisms for systemic vascular dysfunction, a central feature of preeclampsia.
Pathophysiology-oriented diagnostic criteria might improve the prediction of adverse maternal and neonatal outcomes, and pathophysiology-oriented management strategies might improve maternal and neonatal outcomes by decreasing the incidence of preterm delivery without increasing adverse maternal outcomes. Further research has the potential to reveal novel therapeutic and preventive strategies.
References
Cunningham FD . Hypertensive Disorders, Williams Obstetrics 24th edn. McGraw-Hill Education. 2014, pp 728–779.
Sibai B, Dekker G, Kupferminc M . Preeclampsia. Lancet 2005; 365: 785–799.
Wenger NK . Recognizing pregnancy-associated cardiovascular risk factors. Am J Cardiol 2014; 113: 406–409.
Veerbeek JH, Hermes W, Breimer AY, van Rijn BB, Koenen SV, Mol BW, Franx A, de Groot CJ, Koster MP . Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension 2015; 65: 600–606.
Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V . Pre-eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol 2014; 63: 1815–1822.
Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK . Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol 1989; 161: 1200–1204.
Sircar M, Thadhani R, Karumanchi SA . Pathogenesis of preeclampsia. Curr Opin Nephrol Hypertens 2015; 24: 131–138.
Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R . Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol 2014; 10: 466–480.
Redman CW, Sargent IL . Latest advances in understanding preeclampsia. Science 2005; 308: 1592–1594.
Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA . Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003; 111: 649–658.
Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA . Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004; 350: 672–683.
Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D'Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA . Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 2006; 12: 642–649.
Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA, CPEP Study Group. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med 2006; 355: 992–1005.
Sibai BM, Stella CL . Diagnosis and management of atypical preeclampsia-eclampsia. Am J Obstet Gynecol 2009; 200: 481.e1–7.
Stella CL, Malik KM, Sibai BM . HELLP syndrome: an atypical presentation. Am J Obstet Gynecol 2008; 198: e6–e8.
Ohno Y, Terauchi M, Tamakoshi K, Shiozaki A, Saito S . The risk factors for labor onset hypertension. Hypertens Res 2016; 39: 260–265.
Puttapitakpong P, Phupong V . Combination of serum angiopoietin-2 and uterine artery Doppler for prediction of preeclampsia. Hypertens Res 2016; 39: 95–99.
Taebi M, Sadat Z, Saberi F, Kalahroudi MA . Early pregnancy waist-to-hip ratio and risk of preeclampsia: a prospective cohort study. Hypertens Res 2015; 38: 80–83.
Roberts JM, Hubel CA . The two stage model of preeclampsia: variations on the theme. Placenta 2009; 30 (Suppl A): S32–S37.
De Wolf F, De Wolf-Peeters C, Brosens I, Robertson WB . The human placental bed: electron microscopic study of trophoblastic invasion of spiral arteries. Am J Obstet Gynecol 1980; 137: 58–70.
Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH . Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest 1997; 99: 2139–2151.
Brosens IA, Robertson WB, Dixon HG . The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu 1972; 1: 177–191.
Robertson WB, Brosens I, Dixon HG . The pathological response of the vessels of the placental bed to hypertensive pregnancy. J Pathol Bacteriol 1967; 93: 581–592.
Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, Vercruysse L, van Assche A . Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol 1991; 98: 648–655.
Zhou Y, Damsky CH, Chiu K, Roberts JM, Fisher SJ . Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J Clin Invest 1993; 91: 950–960.
Saito S, Nakashima A, Shima T, Ito M . Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol 2010; 63: 601–610.
Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y . Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab 2003; 88: 2348–2351.
Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, Cabrol D, Frankenne F, Foidart JM . Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab 2003; 88: 5555–5563.
Kendall RL, Thomas KA . Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA 1993; 90: 10705–10709.
Mustonen T, Alitalo K . Endothelial receptor tyrosine kinases involved in angiogenesis. J Cell Biol 1995; 129: 895–898.
Clark DE, Smith SK, He Y, Day KA, Licence DR, Corps AN, Lammoglia R, Charnock-Jones DS . A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol Reprod 1998; 59: 1540–1548.
Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J . Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest 1989; 84: 1470–1478.
Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, Nguyen HV, Aiello LM, Ferrara N, King GL . Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994; 331: 1480–1487.
Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N . Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989; 246: 1306–1309.
Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT . Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science 1989; 246: 1309–1312.
Kumasawa K, Ikawa M, Kidoya H, Hasuwa H, Saito-Fujita T, Morioka Y, Takakura N, Kimura T, Okabe M . Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci USA 2011; 108: 1451–1455.
Zhu X, Wu S, Dahut WL, Parikh CR . Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis 2007; 49: 186–193.
Eskens FA, Verweij J . The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors; a review. Eur J Cancer 2006; 42: 3127–3139.
Nagamatsu T, Fujii T, Kusumi M, Zou L, Yamashita T, Osuga Y, Momoeda M, Kozuma S, Taketani Y . Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology 2004; 145: 4838–4845.
Fan X, Rai A, Kambham N, Sung JF, Singh N, Petitt M, Dhal S, Agrawal R, Sutton RE, Druzin ML, Gambhir SS, Ambati BK, Cross JC, Nayak NR . Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications. J Clin Invest 2014; 124: 4941–4952.
Sela S, Natanson-Yaron S, Zcharia E, Vlodavsky I, Yagel S, Keshet E . Local retention versus systemic release of soluble VEGF receptor-1 are mediated by heparin-binding and regulated by heparanase. Circ Res 2011; 108: 1063–1070.
Miyake T, Kumasawa K, Sato N, Takiuchi T, Nakamura H, Kimura T . Soluble VEGF receptor 1 (sFLT1) induces non-apoptotic death in ovarian and colorectal cancer cells. Sci Rep 2016; 6: 24853.
Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, Gomez R, Edwin S, Chaiworapongsa T, Levine RJ, Karumanchi SA . A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med 2008; 21: 9–23.
Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ . Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation 2010; 122: 478–487.
Mashini IS, Albazzaz SJ, Fadel HE, Abdulla AM, Hadi HA, Harp R, Devoe LD . Serial noninvasive evaluation of cardiovascular hemodynamics during pregnancy. Am J Obstet Gynecol 1987; 156: 1208–1213.
Robson SC, Hunter S, Boys RJ, Dunlop W . Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol 1989; 256: H1060–H1065.
Duvekot JJ, Cheriex EC, Pieters FA, Menheere PP, Peeters LH . Early pregnancy changes in hemodynamics and volume homeostasis are consecutive adjustments triggered by a primary fall in systemic vascular tone. Am J Obstet Gynecol 1993; 169: 1382–1392.
Mabie WC, DiSessa TG, Crocker LG, Sibai BM, Arheart KL . A longitudinal study of cardiac output in normal human pregnancy. Am J Obstet Gynecol 1994; 170: 849–856.
Christianson RE . Studies on blood pressure during pregnancy. I. Influence of parity and age. Am J Obstet Gynecol 1976; 125: 509–513.
Moutquin JM, Rainville C, Giroux L, Raynauld P, Amyot G, Bilodeau R, Pelland N . A prospective study of blood pressure in pregnancy: prediction of preeclampsia. Am J Obstet Gynecol 1985; 151: 191–196.
Cornelis T, Odutayo A, Keunen J, Hladunewich M . The kidney in normal pregnancy and preeclampsia. Semin Nephrol 2011; 31: 4–14.
Mattot V, Moons L, Lupu F, Chernavvsky D, Gómez RA, Collen D, Carmeliet P . Loss of the VEGF(164) and VEGF(188) isoforms impairs postnatal glomerular angiogenesis and renal arteriogenesis in mice. J Am Soc Nephrol 2002; 13: 1548–1560.
Eremina V, Quaggin SE . The role of VEGF-A in glomerular development and function. Curr Opin Nephrol Hypertens 2004; 13: 9–15.
Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE . Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 2003; 111: 707–716.
Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE . VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 2008; 358: 1129–1136.
Tomiyama H, Yamashina A . Non-invasive vascular function tests: their pathophysiological background and clinical application. Circ J 2010; 74: 24–33.
McEniery CM, Wallace S, Mackenzie IS, McDonnell B, Yasmin, Newby DE, Cockcroft JR, Wilkinson IB . Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension 2006; 48: 602–608.
Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G . Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension 2011; 57: 363–369.
Ras RT, Streppel MT, Draijer R, Zock PL . Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol 2013; 168: 344–351.
Dørup I, Skajaa K, Sørensen KE . Normal pregnancy is associated with enhanced endothelium-dependent flow-mediated vasodilation. Am J Physiol 1999; 276 (3 Pt 2): H821–H825.
Weissgerber TL, Milic NM, Milin-Lazovic JS, Garovic VD . Impaired flow-mediated dilation before, during, and after preeclampsia: a systematic review and meta-analysis. Hypertension 2016; 67: 415–423.
Kelly R, Hayward CS, Avolio A, O’Rourke MF . Non-invasive registration of the arterial pressure pulse waveform using high-fidelity applanation tonometry. J Vasc Med Biol 1989; 1: 142–149.
Nichols WW . Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens 2005; 18: 3S–10S.
Nichols WW, Singh BM . Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol 2002; 17: 543–551.
McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR, ACCT Investigators. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol 2005; 46: 1753–1760.
Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O'Rourke M CAFE Investigators, Anglo-Scandinavian Cardiac Outcomes Trial Investigators, CAFE Steering Committee and Writing Committee. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 2006; 113: 1213–1225.
Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV . Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 2007; 50: 197–203.
Chirinos JA, Zambrano JP, Chakko S, Veerani A, Schob A, Willens HJ, Perez G, Mendez AJ . Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease. Hypertension 2005; 45: 980–985.
London GM, Blacher J, Pannier B, Guérin AP, Marchais SJ, Safar ME . Arterial wave reflections and survival in end-stage renal failure. Hypertension 2001; 38: 434–438.
Fujime M, Tomimatsu T, Okaue Y, Koyama S, Kanagawa T, Taniguchi T, Kimura T . Central aortic blood pressure and Augmentation index during normal pregnancy. Hypertens Res 2012; 35: 633–638.
Macedo ML, Luminoso D, Savvidou MD, McEniery CM, Nicolaides KH . Maternal wave reflections and arterial stiffness in normal pregnancy as assessed by applanation tonometry. Hypertension 2008; 51: 1047–1051.
Khalil A, Jauniaux E, Cooper D, Harrington K . Pulse wave analysis in normal pregnancy: a prospective longitudinal study. PLoS ONE 2009; 4: e6134.
Spasojevic M, Smith SA, Morris JM, Gallery ED . Peripheral arterial pulse wave analysis in women with pre-eclampsia and gestational hypertension. BJOG 2005; 112: 1475–1478.
Khalil A, Jauniaux E, Harrington K . Antihypertensive therapy and central hemodynamics in women with hypertensive disorders in pregnancy. Obstet Gynecol 2009; 113: 646–654.
Khalil AA, Cooper DJ, Harrington KF . Pulse wave analysis: a preliminary study of a novel technique for the prediction of pre-eclampsia. BJOG 2009; 116: 268–276.
Khalil A, Akolekar R, Syngelaki A, Elkhouli M, Nicolaides KH . Maternal hemodynamics at 11-13 weeks' gestation and risk of pre-eclampsia. Ultrasound Obstet Gynecol 2012; 40: 28–34.
Tomimatsu T, Fujime M, Kanayama T, Mimura K, Koyama S, Kanagawa T, Kimura T . Maternal arterial stiffness in normotensive pregnant women who subsequently deliver babies that are small for gestational age. Eur J Obstet Gynecol Reprod Biol 2013; 169: 24–27.
Tomimatsu T, Fujime M, Kanayama T, Mimura K, Koyama S, Kanagawa T, Endo M, Shimoya K, Kimura T . Abnormal pressure-wave reflection in pregnant women with chronic hypertension: association with maternal and fetal outcomes. Hypertens Res 2014; 37: 989–992.
Rogers DT, Colon M, Gambala C, Wilkins I, Hibbard JU . Effects of magnesium on central arterial compliance in preeclampsia. Am J Obstet Gynecol 2010; 202: 448.e1–8.
Yinon Y, Kingdom JC, Odutayo A, Moineddin R, Drewlo S, Lai V, Cherney DZ, Hladunewich MA . Vascular dysfunction in women with a history of preeclampsia and intrauterine growth restriction: insights into future vascular risk. Circulation 2010; 122: 1846–1853.
Asmar R Pulse wave velocity: principle and measurement. In Asmar R (ed.). Arterial Stiffness and Pulse Wave Velocity. Amsterdam: Elsevier. 1999, pp 25–56.
Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J . Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 2006; 113: 664–670.
Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S . Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension 2002; 39: 10–15.
Oyama-Kato M, Ohmichi M, Takahashi K, Suzuki S, Henmi N, Yokoyama Y, Kurachi H . Change in pulse wave velocity throughout normal pregnancy and its value in predicting pregnancy-induced hypertension: a longitudinal study. Am J Obstet Gynecol 2006; 195: 464–469.
Kaihura C, Savvidou MD, Anderson JM, McEniery CM, Nicolaides KH . Maternal arterial stiffness in pregnancies affected by preeclampsia. Am J Physiol Heart Circ Physiol 2009; 297: H759–H764.
Powe CE, Levine RJ, Karumanchi SA . Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 2011; 123: 2856–2869.
Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Gonçalves L, Gomez R, Edwin S, Mazor M . Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med 2005; 17: 3–18.
Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, Lim KH, Wenger JB, Thadhani R, Karumanchi SA . Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 2012; 125: 911–919.
Kusanovic JP, Romero R, Chaiworapongsa T, Erez O, Mittal P, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Edwin SS, Gomez R, Yeo L, Conde-Agudelo A, Hassan SS . A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med 2009; 22: 1021–1038.
Powers RW, Jeyabalan A, Clifton RG, Van Dorsten P, Hauth JC, Klebanoff MA, Lindheimer MD, Sibai B, Landon M, Miodovnik M Eunice Kennedy Shriver National Institute of Child Health Human Development Maternal-Fetal Medicine Units Network. Soluble fms-Like tyrosine kinase 1 (sFlt1), endoglin and placental growth factor (PlGF) in preeclampsia among high risk pregnancies. PLoS ONE 2010; 5: e13263.
McElrath TF, Lim KH, Pare E, Rich-Edwards J, Pucci D, Troisi R, Parry S . Longitudinal evaluation of predictive value for preeclampsia of circulating angiogenic factors through pregnancy. Am J Obstet Gynecol 2012; 207: 407.e1–7.
Kleinrouweler CE, Wiegerinck MM, Ris-Stalpers C, Bossuyt PM, van der Post JA, von Dadelszen P, Mol BW, Pajkrt E, EBM CONNECT Collaboration. Accuracy of circulating placental growth factor, vascular endothelial growth factor, soluble fms-like tyrosine kinase 1 and soluble endoglin in the prediction of pre-eclampsia: a systematic review and meta-analysis. BJOG 2012; 119: 778–787.
Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, Olovsson M, Brennecke SP, Stepan H, Allegranza D, Dilba P, Schoedl M, Hund M, Verlohren S . Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med 2016; 374: 13–22.
Chaiworapongsa T, Romero R, Savasan ZA, Kusanovic JP, Ogge G, Soto E, Dong Z, Tarca A, Gaurav B, Hassan SS . Maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med 2011; 24: 1187–1207.
Rana S, Schnettler WT, Powe C, Wenger J, Salahuddin S, Cerdeira AS, Verlohren S, Perschel FH, Arany Z, Lim KH, Thadhani R, Karumanchi SA . Clinical characterization and outcomes of preeclampsia with normal angiogenic profile. Hypertens Pregnancy 2013; 32: 189–201.
Verlohren S, Galindo A, Schlembach D, Zeisler H, Herraiz I, Moertl MG, Pape J, Dudenhausen JW, Denk B, Stepan H . An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol 2010; 202: 161.e1–161.e11.
Sunderji S, Gaziano E, Wothe D, Rogers LC, Sibai B, Karumanchi SA, Hodges-Savola C . Automated assays for sVEGF R1 and PlGF as an aid in the diagnosis of preterm preeclampsia: a prospective clinical study. Am J Obstet Gynecol 2010; 202: 40.e1–7.
Perni U, Sison C, Sharma V, Helseth G, Hawfield A, Suthanthiran M, August P . Angiogenic factors in superimposed preeclampsia: a longitudinal study of women with chronic hypertension during pregnancy. Hypertension 2012; 59: 740–746.
Rolfo A, Attini R, Nuzzo AM, Piazzese A, Parisi S, Ferraresi M, Todros T, Piccoli GB . Chronic kidney disease may be differentially diagnosed from preeclampsia by serum biomarkers. Kidney Int 2013; 83: 177–181.
Leaños-Miranda A, Campos-Galicia I, Berumen-Lechuga MG, Molina-Pérez CJ, García-Paleta Y, Isordia-Salas I, Ramírez-Valenzuela KL . Circulating angiogenic factors and the risk of preeclampsia in systemic lupus erythematosus pregnancies. J Rheumatol 2015; 42: 1141–1149.
Qazi U, Lam C, Karumanchi SA, Petri M . Soluble Fms-like tyrosine kinase associated with preeclampsia in pregnancy in systemic lupus erythematosus. J Rheumatol 2008; 35: 631–634.
Young B, Levine RJ, Salahuddin S, Qian C, Lim KH, Karumanchi SA, Rana S . The use of angiogenic biomarkers to differentiate non-HELLP related thrombocytopenia from HELLP syndrome. J Matern Fetal Neonatal Med 2010; 23: 366–370.
Suzuki H, Ohkuchi A, Matsubara S, Takei Y, Murakami M, Shibuya M, Suzuki M, Sato Y . Effect of recombinant placental growth factor 2 on hypertension induced by full-length mouse soluble fms-like tyrosine kinase 1 adenoviral vector in pregnant mice. Hypertension 2009; 54: 1129–1135.
Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP . Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension 2010; 55: 380–385.
Mimura K, Tomimatsu T, Sharentuya N, Tskitishvili E, Kinugasa-Taniguchi Y, Kanagawa T, Kimura T . Nicotine restores endothelial dysfunction caused by excess sFlt1 and sEng in an in vitro model of preeclamptic vascular endothelium: a possible therapeutic role of nicotinic acetylcholine receptor (nAChR) agonists for preeclampsia. Am J Obstet Gynecol 2010; 202 (464): e1–e6.
Kakigano A, Tomimatsu T, Mimura K, Kanayama T, Fujita S, Minato K, Kumasawa K, Taniguchi Y, Kanagawa T, Endo M, Ishihara T, Namba T, Mizushima T, Kimura T . Drug repositioning for preeclampsia therapeutics by in vitro screening: phosphodiesterase-5 inhibitor vardenafil restores endothelial dysfunction via induction of placental growth factor. Reprod Sci 2015; 22: 1272–1280.
Thadhani R, Kisner T, Hagmann H, Bossung V, Noack S, Schaarschmidt W, Jank A, Kribs A, Cornely OA, Kreyssig C, Hemphill L, Rigby AC, Khedkar S, Lindner TH, Mallmann P, Stepan H, Karumanchi SA, Benzing T . Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation 2011; 124: 940–950.
Costantine MM, Cleary K, Hebert MF, Ahmed MS, Brown LM, Ren Z, Easterling TR, Haas DM, Haneline LS, Caritis SN, Venkataramanan R, West H, D'Alton M, Hankins G Eunice Kennedy Shriver National Institute of Child Health and Human Development Obstetric-Fetal Pharmacology Research Units Network. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial. Am J Obstet Gynecol 2016; 214: 720.e1–720.e17.
Smith GC, Pell JP, Walsh D . Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129290 births. Lancet 2001; 357: 2002–2006.
Bellamy L, Casas JP, Hingorani AD, Williams DJ . Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007; 335: 974.
Onoue K, Uemura S, Takeda Y, Somekawa S, Iwama H, Nishida T, Morikawa Y, Nakagawa H, Tsutsumi T, Sung JH, Takemoto Y, Soeda T, Okayama S, Ishigami K, Kawata H, Horii M, Nakajima T, Saito Y . Usefulness of soluble Fms-like tyrosine kinase-1 as a biomarker of acute severe heart failure in patients with acute myocardial infarction. Am J Cardiol 2009; 104: 1478–1483.
Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, Hacker MR, Rhee JS, Mitchell J, Mahmood F, Hess P, Farrell C, Koulisis N, Khankin EV, Burke SD, Tudorache I, Bauersachs J, del Monte F, Hilfiker-Kleiner D, Karumanchi SA, Arany Z . Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 2012; 485: 333–338.
Kvehaugen AS, Dechend R, Ramstad HB, Troisi R, Fugelseth D, Staff AC . Endothelial function and circulating biomarkers are disturbed in women and children after preeclampsia. Hypertension 2011; 58: 63–69.
Akhter T, Wikström AK, Larsson M, Larsson A, Wikström G, Naessen T . Association between angiogenic factors and signs of arterial aging in women with pre-eclampsia. Ultrasound Obstet Gynecol (e-pub ahead of print 3 June 2016; doi:10.1002/uog.15981.
Kurabayashi T, Mizunuma H, Kubota T, Kiyohara Y, Nagai K, Hayashi K . Pregnancy-induced hypertension is associated with maternal history and a risk of cardiovascular disease in later life: Japanese cross-sectional study. Maturitas 2013; 75: 227–231.
Nohira T . Hypertension and metabolic abnormalities later in life after preeclampsia. Hypertens Res Pregnancy 2013; 1: 52–56.
Hosaka M, Asayama K, Staessen JA, Tatsuta N, Satoh M, Kikuya M, Ohkubo T, Satoh H, Imai Y, Nakai K . Relationship between maternal gestational hypertension and home blood pressure in 7-year-old children and their mothers: Tohoku Study of Child Development. Hypertens Res 2015; 38: 776–782.
Watanabe K, Kimura C, Iwasaki A, Mori T, Matsushita H, Shinohara K, Wakatsuki A, Gosho M, Miyano I . Pregnancy-induced hypertension is associated with an increase in the prevalence of cardiovascular disease risk factors in Japanese women. Menopause 2015; 22: 656–659.
Berks D, Hoedjes M, Raat H, Duvekot JJ, Steegers EA, Habbema JD . Risk of cardiovascular disease after pre-eclampsia and the effect of lifestyle interventions: a literature-based study. BJOG 2013; 120: 924–931.
Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Piña IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D'Armiento J, Kris-Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC Jr, Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V, Wenger NK . Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation 2011; 123: 1243–1262.
Takagi K, Yamasaki M, Nakamoto O, Saito S, Suzuki H, Seki H, Takeda S, Ohno Y, Sugimura M, Suzuki Y, Watanabe K, Matsubara K, Makino S, Metoki H, Yamamoto T . A review of Best Practice Guide 2015 for care and treatment of hypertension in pregnancy. Hypertens Res Pregnancy 2015; 3: 65–103.
Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S, Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res 2014; 37: 253–390.
Acknowledgements
This study was supported in part by Grant-in-Aid for General Scientific Research (26462506, 26670724, 25293342, 26462488, 15H02564 and 15K10669) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tomimatsu, T., Mimura, K., Endo, M. et al. Pathophysiology of preeclampsia: an angiogenic imbalance and long-lasting systemic vascular dysfunction. Hypertens Res 40, 305–310 (2017). https://doi.org/10.1038/hr.2016.152
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2016.152
Keywords
This article is cited by
-
An integral role of mitochondrial function in the pathophysiology of preeclampsia
Molecular Biology Reports (2024)
-
Matrine promotes trophoblast invasion and reduces inflammation via miR-19a-3p to prevent preeclampsia
Molecular & Cellular Toxicology (2023)
-
First Trimester CD93 as a Novel Marker of Preeclampsia and Its Complications: A Pilot Study
High Blood Pressure & Cardiovascular Prevention (2023)
-
Beta-Blockers in Pregnancy: Clinical Update
Current Hypertension Reports (2023)
-
Establishment of a nomogram model for predicting adverse outcomes in advanced-age pregnant women with preterm preeclampsia
BMC Pregnancy and Childbirth (2022)