Abstract
Primary aldosteronism (PA) is the most common secondary cause of hypertension. The present study investigated differences in left ventricular structure and function between hypertensive patients with PA and sucjects with essential hypertension (EH). One hundred patients with PA and 100 controls with EH were matched for age, gender, and 24-h ambulatory monitoring blood pressure (BP). Left ventricular mass index (LVMI), left atrial volume index (LAVI) and ejection fraction were calculated. LV diastolic function was estimated as the ratio of the early diastolic velocities (E) from transmitral inflow to the early diastolic velocities (e′) of tissue Doppler at mitral annulus. PA and EH patients had similar LV dimensions, LV wall thicknesses, LVMI and LV systolic function. PA was associated with greater impairment in diastolic function, as reflected by the lower e′ (P=0.004), higher E/e′ ratio (P=0.005) and higher LAVI (P=0.02). The LV geometric dimensions and patterns of LV hypertrophy were similar between male patients from the PA and EH groups. However, in female patients, PA was correlated with higher LV internal dimensions (P=0.001), higher LVMI (P=0.04) and lower relative wall thickness (RWT, P=0.001). Multivariate analysis showed that LV diastolic function was independently correlated with age (β=0.416, P<0.001), 24-h systolic BP (β=0.238, P=0.016) and serum potassium (β=−0.201, P=0.036) in PA patients. In conclusion, PA appears to contribute to the impairment of LV diastolic function in both sexes as well as the higher prevalence of eccentric hypertrophy in women than in men compared with EH. Age, 24-h systolic BP and serum potassium levels are independent risk factors for LV diastolic function in PA patients.
Similar content being viewed by others
Introduction
For many years, primary aldosteronism (PA) has been considered to be a rare cause of hypertension affecting ~1% of the hypertensive population. However, recent developments in diagnostic screening tests for the measurement of the aldosterone-to-renin ratio (ARR) and its accurate localization by adrenal venous sampling have highlighted a higher incidence of PA (5–13% of all unselected hypertensive patients) than previously thought. Pa is now known to be the most common secondary cause of hypertension.1, 2, 3, 4, 5
One of the most severe target organ damages of hypertension is left ventricular hypertrophy (LVH), which is a strong predictor of adverse prognosis.6 A spectrum of LV geometric adaptations may occur during arterial hypertension due to systemic hemodynamics and ventricular load, including concentric hypertrophy, eccentric hypertrophy and concentric remodeling.7 In addition to the hemodynamic component, the renin–angiotensin–aldosterone system is involved in the process of LVH.8, 9 However, the relationship between plasma aldosterone concentrations and LV geometry appears to be more complex than originally thought, as demonstrated by the Framingham Offspring Study, which has revealed that plasma aldosterone concentrations are positively correlated with concentric left ventricular remodeling and inversely correlated with left ventricular diastolic dimensions in women but not in men.10 Several studies have shown a higher prevalence of LVH in patients with PA than in those with essential hypertension (EH).11, 12 However, in a two-center, case–control study, patients with PA have been found to have similar LV geometry and systolic function compared with those of EH patients matched for age, gender and blood pressure (BP).13 Chronic elevation of circulating aldosterone is associated with myocardial fibrosis, which may initially adversely alter LV diastolic function and ultimately systolic ventricular function.14, 15 Because excessive serum aldosterone may influence serum potassium, in one study, 9–37% of patients with PA had hypokalemia,16 and several studies have demonstrated that serum potassium is significantly associated with higher cardiovascular morbidity and LVH.17, 18 However, it is still unclear whether PA has a pathogenic role in LV geometry and function, and whether serum potassium is associated with these changes or whether PA leads to different outcomes in both sexes beyond elevated BP alone.
Therefore, we consider it worthwhile to investigate the inappropriateness of LV geometric and systolic and diastolic function in patients with PA, as well as other risk factors, particularly the serum potassium level, and to evaluate the sex-specific relationship between serum aldosterone and echocardiographic indices of cardiac structure and function.
Materials and methods
Subjects
A total of 100 patients diagnosed with PA aged 14 to 74 years (58 males and 42 females) were enrolled in the study from March 2009 to August 2014. Sixty-six patients had aldosterone-producing adenomas, and 34 had bilateral adrenal hyperplasia. Patients with atrial fibrillation, systolic left ventricular dysfunction, chronic heart failure, severe arrhythmia, myocardial infarction, cardiomyopathy or valvular disorders were excluded from the study. The basal evaluation was performed after discontinuation of any antihypertensive treatment, except for calcium-channel and adrenoreceptor blockers, for 4 weeks. Patients with hypokalemia were given adequate oral potassium supplements before the hormonal evaluations. The ARR was used with a cutoff level of 24 ng dl−1 per ng ml−1 per h plus an aldosterone level >20 ng dl−1.19 A positive saline infusion test (that is, posttest aldosterone levels >10 ng dl−1)16 was considered a confirmatory test. In addition, computed tomography and/or magnetic resonance of the adrenal glands were used as imaging techniques.16
Patients with PA were carefully matched for age, gender, body size, clinic BP values and 24-h ambulatory monitoring BP values at the time of the basal examination. One hundred patients diagnosed with EH were referred to our inpatient ward for a high BP diagnostic workup, including supine and standing plasma renin activity and aldosterone measurements from the hypertension department in Ruijin Hospital, Shanghai, China. Prior informed consent was obtained from all patients for participation in the study, and all participants provided written informed consent. This study was approved by the Ethics Committee of Ruijin Hospital.
BP Measurement
Validated oscillometric SpaceLabs 90217 monitors (Space-Labs, Redmond, WA, USA) were programmed to obtain BP readings at 20-min intervals from 0600 h to 2200 h and at 30-min intervals from 2200 h to 0600 h.
Echocardiography
Comprehensive transthoracic echocardiography was performed using a cardiac ultrasound system (iE33 ultrasound system, Royal Dutch Philips Electronics, Bothell, WA, USA). The LV diameters, interventricular septal wall and posterior wall thickness, were measured at end-diastole from M-mode recordings according to the guidelines of the American Society of Echocardiography.20 The relative wall thickness (RWT) was calculated as the ratio of posterior wall thickness to one-half of the LV internal dimension, thus permitting categorization of an increase in LV mass index (LVMI) as either concentric (RWT⩾0.42) or eccentric (RWT⩽0.42) hypertrophy and allows identification of concentric remodeling (normal LVMI with increased RWT).21 The ejection fraction and fractional shortening were calculated by standard quantification methods with M-mode measurements from the two-dimensional image.22 LV end-diastolic and end-systolic volumes were measured at end-diastole and end-systole from M-mode recordings and calculated with the Teicholz’s correction of the cube formula.23, 24 The LV mass was calculated from linear measurements derived from M-mode echocardiograms according to the formula described by Devereux et al.25 In this study, we used BSA (body surface area) as the indexing term of LV mass, and LVMI is reported as g m−2 BSA. We used the biplane disk summation method to calculate left atrial (LA) volumes in the four-chamber views, and these values were indexed by BSA as LA volume index (LAVI) in g m−2 BSA.
The peak early filling (E-wave) and late diastolic filling (A-wave) transmitral flow velocities, the E/A ratio, and the deceleration time of the early filling velocity were assessed by pulsed-wave Doppler in the apical four-chamber. The isovolumic relaxation time was derived by placing the cursor of the CW Doppler in the LV outflow tract at the onset of mitral inflow.24 Pulsed-wave tissue Doppler imaging (TDI) was performed by means of mitral annulus in four-chamber apical views. The early diastolic annular velocity (e′) was measured by TDI recordings. The ratio of the mitral inflow E velocity to tissue Doppler e′ (E/e′) was calculated.26 For the assessment of global LV diastolic function, tissue Doppler signals at the septal and lateral sides of the mitral annulus were acquired, and their average values were calculated.26
To calculate the intra-observer variability, the parameters were reassessed in the first 20 patients by the same observer 4 weeks after the first evaluation, and the observer did not review the patients’ previous reports.
Statistical analysis
Data were stored and analyzed using the SPSS 13.0 statistical package (SPSS, Chicago, IL, USA). Continuous data are expressed as the mean±s.d. and categorical variables as a percentage of the group. Logarithmically transformed values of skewed variables were used for analysis. Independent t-tests were performed to determine the differences between studied groups, and the χ2-test was used for comparisons of proportions among groups. Pearson’s or Spearman’s correlation analysis was performed to assess the association between the E/e′ ratio and other risk factors. Multivariate linear regression analysis was performed using age, sex, hypertension course, diabetes mellitus, 24-h systolic BP (SBP) and diastolic BP (DBP), 24-h heart rate, serum creatinine, serum and urinary potassium, serum sodium, plasma aldosterone, active plasma rennin and the history of antihypertensive agent use (4 weeks before) including mineralocorticoid receptor inhibitor as independent variables, with the E/e′ ratio as the dependent variable in the model. The multivariate linear regression equation was forward selected, and this was followed by backward elimination of covariates, thus resulting in an equation in which only covariates that significantly increase the predictability of the dependent variable were included. All covariates included in the final model were tested for interactions with each other. A P-value of <0.05 was defined as statistically significant and a P-value of <0.01 was defined as highly significant.
Results
Characteristics of patients with PA and EH
Clinical and biological data of the PA patients and EH controls are summarized in Table 1. The cases and controls were similar in age (50±12 years), gender distribution (58% male), hypertension course (10.3±7.0 vs. 10.4±10.0 years), 24-h systolic BP (138±16 vs. 135±14 mm Hg) and 24-h DBP (87±9 vs. 85±10 mm Hg). Furthermore, 24-h ambulatory monitoring showed that the night-time ambulatory SBP was higher in PA subjects than in EH subjects (132±19 vs. 126±17 mm Hg, P=0.02), whereas the day-time BP and night-time DBP were similar in these two groups. Heart rate, pulse pressure and clinic BP were also similar between the PA and EH subjects. Of the remaining parameters, serum total cholesterol, triglycerides and LDL-C were significantly higher in the EH group than in the PA group (TC: 4.8±0.9 vs. 4.4±0.9 mmol l−1, P=0.001; TG: 2.4±2.1 vs. 1.7±1.0 mmol l−1, P=0.004; LDL-C: 3.0±0.8 vs. 2.6±0.7 mmol l−1, P=0.005). In addition, serum creatinine and uric acid were higher in the EH group than in the PA group (serum creatinine: 81±23 vs. 74±21 μmol l−1, P=0.02; uric acid: 347±94 vs. 312±96 μmol l−1, P=0.01). Patients with PA had higher levels of N-terminal pro-brain natriuretic peptide (NT-proBNP) than did those with EH (lg NT-proBNP: 1.75±0.46 vs. 1.47±0.54, P<0.001). There was no difference in incidence of diabetes, fasting glucose and 2-h glucose between these two groups.
As expected, patients with PA had lower serum potassium than did the EH controls (3.21±0.43 vs. 3.82±0.31 mmol l−1, P<0.001), as well as higher serum sodium (141.6±3.5 vs. 139.9±3.1 mmol, P<0.001) and significantly higher urinary potassium (63.4±31.8 vs. 31.3±12.9 mmol per 24 h, P<0.001). The percentage of patients affected by hypokalemia in the PA group was 76%. Similarly, plasma aldosterone (34.6±24.3 vs. 18.0±10.7 ng dl−1, P<0.001), ARR (lg ARR: 1.84±0.64 vs. 0.95±0.65, P<0.001) and urinary aldosterone (22.8±14.3 vs. 7.5±6.8 μg per 24 h, P<0.001) were significantly higher in the PA group than in the EH controls, whereas plasma renin activity (0.87±1.04 vs. 3.18±2.56 ng ml−1 per h, P<0.001) was significantly lower (Table 1).
Ninety-three patients received calcium-channel blockers and 48 patients received adrenoceptor blockers in the PA group. In the EH group, 33 patients received diuretics, 27 patients received beta-blockers, 28 patients received angiotensin-converting enzyme inhibitors, 36 patients received angiotensin receptor blockers, 80 patients received calcium-channel blockers and 10 patients received adrenoceptor blockers. The prescription history of antihypertensive medication before study enrollment is shown in Table 1. The usage of mineralocorticoid receptor inhibitors and adrenoceptor blockers was higher (29 vs. 3%, P<0.001; 10 vs. 3%, P=0.045; respectively) in the PA group than in the EH controls, whereas there was no difference in the usage of calcium-channel blocker, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, beta-blockers or diuretics (Table 1).
Echocardiographic data of PA and EH
The echocardiographic measurements were performed by the same well-trained sonographer and the intra-observer variability was assessed prior to the study. The variation coefficients of LVMI, LAVI, E/A, deceleration time, isovolumic relaxation time and E/e′ were 7.2%, 6.1%, 8.4%, 8.3%, 6.2% and 6.3%, respectively.
Echocardiographic variables are compared between the PA patients and EH controls in Table 2. The LV dimensions and wall thickness (including end-diastolic RWT) were similar between the PA and EH patients. Moreover, LV mass, LVMI and LV volumes were similar in PA and EH patients. Patterns of LV geometric adaptations and systolic function, measured as ejection fraction, were statistically similar in both patient groups.
The parameters of left ventricular diastolic function between the PA patients and EH controls are shown in Table 2. PA patients had greater impairment in diastolic function, as reflected by lower e′ (5.8±1.9 vs. 6.6±2.1 cm s−1, P=0.004), higher E/e′ ratio (13.5±4.3 vs. 11.9±3.3, P=0.005) and higher LAVIs (23.0±5.8 vs. 21.1±5.5 ml m−2, P=0.02), whereas the early:late-wave ratio, E-wave deceleration time, and isovolumic relaxation time did not differ between the groups. The prevalence of diastolic dysfunction, diagnosed according to the European Society of Cardiology guidelines,27 was significantly greater in patients with PA than in EH subjects (28 vs. 14%, P=0.015).
After adjustment for the prescription history of antihypertensive agents before study enrollment, including mineralocorticoid receptor inhibitors, the echocardiographic results were similar. PA patients had lower e′ (5.8±0.2 vs. 6.6±0.2 cm s−1, P=0.007), higher E/e′ ratio (11.6±0.4 vs. 11.4±0.3, P=0.02) and higher LAVIs (23.0±0.6 vs. 21.0±0.6 ml m−2, P=0.03) compared with those of the EH controls, whereas the LV geometric parameters and systolic function were statistically similar between both groups (data not shown).
Gender differences in echocardiographic parameters of PA and EH
Clinical characteristics and echocardiographic parameters were further stratified by gender in PA and EH patients (Table 3). There was no difference in age, body mass index, hypertension course, clinic BP (data not shown), 24-h BP and heart rate between sex-specific PA and EH groups. Furthermore, 24-h ambulatory monitoring showed that there was no difference in day-time and night-time BP between male PA and EH patients. However, the 24-h DBP and night-time SBP were higher in female PA patients than in female EH subjects (84±8 vs. 80±7 mm Hg, P=0.03 and 128±19 vs. 119±13 mm Hg, P=0.02; respectively), whereas the 24-h SBP, day-time BP and night-time DBP were similar between these two groups. Male patients with PA had lower serum potassium (3.28±0.44 vs. 3.78±0.29 mmol l−1, P<0.001), higher urinary potassium (58.2±29.4 vs. 32.0±13.1 mmol per 24 h, P<0.001), higher serum sodium (141.4±3.4 vs. 138.8±3.3 mmol l−1, P<0.001), higher plasma aldosterone (33.8±22.3 vs. 15.5±8.4 pg ml−1, P<0.001), higher urinary aldosterone (23.0±14.0 vs. 7.8±8.3 μg per 24 h, P<0.001), lower plasma renin activity (0.99±1.06 vs. 3.26±2.61 ng l−1 per h, P<0.001) and higher ARRs (lg ARR: 1.72±0.53 vs. 0.93±0.77, P<0.001) than did the EH controls. Similar results were found in female PA patients and EH controls. In addition, female patients with PA had higher urinary sodium and plasma NT-proBNP than did EH controls (150.9±63.7 vs. 118.2±55.6 mmol per 24 h, P=0.02 and lgNT-proBNP: 1.86±0.42 vs. 1.45±0.52, P<0.001; respectively). Female PA patients had relatively higher lg ARR than did male PA patients (3.00±0.74 vs. 2.72±0.53, P<0.05; Table 3)
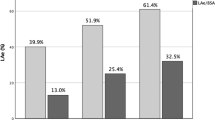
The left ventricular geometric and systolic function parameters, including LVMI, RWT and patterns of LV hypertrophy, were similar among male patients of the PA and EH groups. However, male PA patients had lower e′ (5.6±1.8 vs. 6.4±1.7 cm s−1, P=0.02) and higher E/e′ ratios (13.9±4.7 vs. 12.2±3.7, P=0.03) than did male EH patients. Otherwise, in female patients, PA was correlated with higher LV end-diastolic diameter and volume (49.0±3.1 vs. 46.5±3.5 mm, P=0.001; 114±17 vs. 101±18 ml, P=0.001, respectively), LV end-systolic diameter and volume (31.0±2.8 vs. 29.2±2.8 mm, P=0.004; 38±9 vs. 33±8 ml, P=0.005, respectively), higher LVMI (103±24 vs. 93±18 g m−2, P=0.04) and lower RWT (0.38±0.05 vs. 0.42±0.04, P=0.001). The prevalence of LV eccentric hypertrophy was higher in female patients with PA than in female patients with EH (36 vs. 19%, P=0.007), and the prevalence of LV concentric remodeling was lower (2 vs. 19%, P<0.001). Female PA patients had greater impairment in diastolic function than did EH controls as reflected by e′ (6.0±2.1 vs. 6.9±2.6 cm s−1, P=0.04), E/e′ ratio (12.8±3.6 vs. 11.6±2.6, P=0.04) and higher LAVIs (22.2±5.9 vs. 19.5±4.4 ml m−2, P=0.02). After adjusting for the prescription history of antihypertensive agents before study enrollment, including mineralocorticoid receptor inhibitors, the echocardiographic results were similar to those above (data not shown).
In both the PA and EH groups, women had lower 24-h BP, lower LV dimensions, volume and wall thickness, and lower LVMI than did men. LV concentric remodeling and hypertrophy were more prevalent in male patients than in female patients with PA (9 vs. 2%, P=0.03 and 40 vs. 26%, P=0.04; respectively). Meanwhile, female PA patients had lower RWT than males (0.38±0.05 vs. 0.43±0.06, P=0.001). In the EH group, the patterns of adaptive LV hypertrophy were similar in both sexes, and women had lower LAVIs (19.5±4.4 vs. 22.1±5.9 ml m−2, P=0.02) than men. Both sexes had similar e′ and E/e′ ratios in the PA and EH groups.
The risk factors for LV diastolic function in PA patients
In PA patients, univariate analysis showed that the E/e′ ratio was directly related to age (r=0.483, P<0.001), hypertension course (r=0.217, P=0.017), diabetes (r=0.129, P=0.018) and 24-h systolic BP (r=0.348, P<0.001), whereas it was inversely related to serum potassium (r=−0.211, P=0.007) as shown in Table 4. LV diastolic function ratio was unrelated to sex, body mass index, 24-h DBP, 24-h heart rate, serum creatinine, urinary potassium, plasma urinary aldosterone, plasma renin activity, serum sodium and prescription history of antihypertensive medication before study enrollment. Meanwhile in multivariate analysis, the E/e′ ratio only correlated with age (β=0.416, P<0.001), 24-h systolic BP (β=0.238, P=0.016) and serum potassium (β=−0.201, P=0.036; Table 4).
The relationship between serum potassium and LV diastolic function in PA is also shown in Figure 1. After adjusting for age, sex, diabetes, hypertension course, body mass index, serum creatinine, plasma aldosterone, plasma renin activity, serum sodium, serum and urinary potassium, 24-h heart rate, 24-h SBP and DBP, serum potassium was significantly correlated with septal and lateral E/e′, and the correlation coefficients were −0.246 (P=0.03) and −0.313 (P=0.009), respectively.
The relationship between serum potassium and left ventricular diastolic function in primary aldosteronism. Serum potassium was significantly correlated with septal and lateral E/e′, the correlation coefficient was −0.246 (P=0.03) and −0.313 (P=0.009), after adjusting for age, sex, diabetes, hypertension course, body mass index, serum creatinine, plasma aldosterone, active plasma renin, serum sodium, serum and urinary potassium, 24-h heart rate, 24-h SBP and DBP (left).
Comparison between subtypes of PA
Of the 100 patients with PA, 66 had adenomas and 34 had bilateral adrenal hyperplasia. The two subgroups were similar in age, BP, serum lipid, glucose, serum and urinary potassium, plasma and urinary aldosterone, plasma renin activity and ARR (data not shown). There was no difference in left ventricular geometry, systolic function or diastolic function between these two subgroups (data not shown).
Adrenal vein sampling and surgery results
Adrenal vein sampling (AVS) was performed in 46 patients. The results showed that 16 patients were left selective, and 11 patients were right selective, whereas 19 patients were without bilateral selection. Among the 27 bilaterally selective patients, 24 patients underwent surgery. In the patients with bilateral selection compared with those without selection by AVS, there were no differences in serum and urinary potassium, plasma and urinary aldosterone, plasma renin activity and the ARR, and there were no differences in left ventricular geometry, systolic function or diastolic function between these two subgroups (data not shown).
In addition, 18 patients underwent surgery without AVS and were diagnosed with adenomas via surgical pathology analysis.
Discussion
In this study, we used echocardiography to compare LV geometry and function in hypertensive patients with PA to equally hypertensive patients with EH. The principal finding was that PA patients had greater impairment in LV diastolic function than did EH patients (both men and women). Serum potassium level was an independent risk factor for LV diastolic function in PA patients. Moreover, PA led to a higher prevalence of eccentric hypertrophy (assessed as higher LV internal dimensions, greater LVMI and lower RWT) than did EH in women but not in men. There were no significant differences in LV wall thickness and LV systolic function in both sexes between the PA and EH groups.
The results of the present study indicate poorer LV diastolic function in PA patients than in the EH controls. Other studies have shown17, 28 that PA patients have a greater impairment of cardiac diastolic function than do essential hypertensive patients. Plasma aldosterone concentration is considered to be the major risk factor for LV hypertrophy and diastolic dysfunction in PA. However, in the present study, we found that age, 24-h systolic BP and serum potassium were independent risk factors for LV diastolic function in PA patients.
TDI has been proven to be superior to conventional Doppler for evaluating diastolic function.28, 29 In the present study, we used conventional Doppler and TDI to evaluate LV diastolic function and found that PA patients and EH subjects had similar conventional Doppler parameters, whereas in TDI analysis, PA patients of both sexes had lower e′ and higher E/e′ values than did patients with EH. Because the LA may become less spherical as it remodels, LA volume has been proposed to be a better index of LA remodeling and may provide superior prognostic information than LA dimension. LAVI has been suggested to be a marker of the severity and duration of diastolic dysfunction.30 We found that PA patients had greater LAVI than did EH subjects, whereas there was no difference in LA dimensions between these two groups. These results confirmed that PA led to poorer LV diastolic function than did EH.
In addition to the conventional robust variables such as age and BP values, serum potassium level was an independent risk factor for LV diastolic function in the present study. In a large PA registry study in Germany, Born-Frontsberg et al.18 have found that PA patients with hypokalemia have a higher cardiovascular morbidity than do those with normokalemia. LV diastolic function is recognized as an early marker of hypertension-related target organ damage and is also a stronger predictor of mortality.30 The present study may provide a possible link for the association between hypokalemia and increased cardiovascular morbidity in PA patients. Srivastava and Young31 have performed an interesting study on LV function in 10 normal volunteers after a 7-day period of potassium depletion and another 7 days of potassium repletion. They have found that moderate potassium depletion impairs LV active relaxation. Several studies have sought to reveal the potential influence of hypokalemia on the mechanical function of the heart and have found that LV active relaxation is significantly impaired in hypokalemia.32, 33 Furthermore, Matsui et al.33 have revealed that hypokalemic rats with impaired LV diastolic function produce more reactive oxygen species and potassium supplementation, which not only reduces the elevated nicotinamide-adenine dinucleotide phosphate oxidase activity but also improves the left ventricular relaxation. Otherwise, potassium supplementation might correct the imbalance of intracellular electrolytes, which is important in mediating cardiac function.34 Hattori et al.35 have also found that excessive aldosterone induces diastolic dysfunction without increasing BP, and is accompanied by increased levels of oxidative stress and inflammation as well as increased expression of genes related to renin–angiotensin–aldosterone system systems. However, the mechanisms of hypokalemia-induced LV diastolic dysfunction are still unclear, and further studies are needed to investigate the detailed mechanisms.
Owing to the robust relationship between serum potassium and LV diastolic function in the present study, LV diastolic function had no association with plasma aldosterone concentration. Because hypokalemia inhibits aldosterone secretion, the hypokalemia patients in our study might have had relatively lower aldosterone levels, because the percentage of hypokalemia (76%) was much higher than the guidelines reported.16 It has been suggested that hypokalemia is associated with LV diastolic dysfunction in conditions with excessive aldosterone. Similar to the results reported by Lin et al.17 indicating that serum potassium levels are significantly associated with LVMI, LVMI had no correlation with plasma aldosterone concentrations in both univariate and multivariate linear models in PA patients.
Whether PA leads to higher LVMI has been controversial because previous studies have shown that patients with PA have greater LVH compared with those with similar BP levels of EH.12, 17, 36 However, in a two-center, case–control study, patients with PA and those with EH have been found to have similar LVMI and systolic function.13 The present study revealed that PA patients, compared with EH controls matched for age, gender, and 24-h ambulatory BP, had similar LVMI. The pattern of increasing LV diastolic dimensions and LVMI, as well as decreasing RWT across serum aldosterone in women, suggests that serum aldosterone is associated with eccentric remodeling, which is consistent with results from previous reports.37 Thus, the present investigation confirms sex-related differences in serum aldosterone as it relates to LVMI and RWT. In the present study, we found that lg ARR was relatively higher in female PA patients than in male PA patients, thus possibly partly explaining the sex-related difference in LV geometry. However, another possible reason for the differences in LV geometric patterns between males and females may be that the 24-h DBP and night-time SBP were higher in female PA patients than in EH subjects. Night-time BP is known to be associated with increased risk of target organ damage and cardiovascular events including death,38, 39 and the higher night-time BP may be partly explained by the higher prevalence of eccentric hypertrophy in female PA subjects. Similar results have been found in the LIFE study40 in which more women have been found to have residual eccentric hypertrophy and LVH, consistently with the results from the community-based Olmsted County Study.41 The potential mechanisms underlying the gender-related differences observed in our study may be partly explained by the molecular expression of beta-myosin heavy chain, ANF mRNA and sarcoplasmic reticulum Ca2+-ATPase mRNA,42 as well as the sex-dependent regulation of fibrosis and inflammation.43 Furthermore, estrogen receptors are present in cardiac fibroblasts and myocytes. It has also been reported that estrogen and aldosterone elicit similar rapid nongenomic responses that use common signaling pathways dependent on protein kinase C as highly specific receptors.44
We found that PA patients and EH subjects had similar LV systolic function results consistent with those from previous reports.13, 17, 45
One limitation of this study is that the follow-up echocardiographic data after appropriate treatments with either medications or surgery in PA patients have not yet been established. In this regard, the potential specificity of PA-induced LV diastolic dysfunction and sex-specific echocardiographic findings cannot be completely discussed in this study. Second, we provided data regarding only hypokalemia and excess aldosterone-induced LV diastolic function, but the effects on cardiovascular mortality or morbidity were not determined in this study. Further long-term follow-up studies are needed to investigate the clinical effects of hypokalemia on cardiovascular outcomes. Third, in the present study, only 46 PA patients underwent AVS, and the success rate was only 24 of 46 (52%) patients, so we must improve this technique to accomplish successful subtype diagnosis.46 Because surgically curable aldosterone-producing adenoma is more liable to cause severe hypertension with higher aldosterone levels and lower serum potassium levels in the clinical course than idiopathic hyperaldosteronism, the earlier and precise diagnosis and treatment of aldosterone-producing adenoma may be beneficial for reducing cardiovascular complications.47 Some cases of idiopathic hyperaldosteronism with unilateral non-functioning adenoma and CT-negative micro-aldosterone-producing adenoma have been reported,48, 49 and therefore, AVS should be recommended for PA patients who require surgery.
In conclusion, our results indicate that PA, compared with EH, contributes to the impairment of LV diastolic function in both sexes, as well as a higher prevalence of eccentric hypertrophy in women than in men. Age, 24-h systolic BP and serum potassium levels are independent risk factors for LV diastolic function in PA patients.
References
Rossi GP, Seccia TM, Pessina AC . Primary aldosteronism—part I: prevalence, screening, and selection of cases for adrenal vein sampling. J Nephrol 2008; 21: 447–454.
Rossi GP . Prevalence and diagnosis of primary aldosteronism. Curr Hypertens Rep 2010; 12: 342–348.
Fardella CE, Mosso L, Gómez-Sánchez C, Cortés P, Soto J, Gómez L, Pinto M, Huete A, Oestreicher E, Foradori A, Montero J . Primary hyperaldostronism in essential hypertensives: prevalence, biochemical profile, and molecular biology. J Clin Endocrinol Metab 2000; 85: 1863–1867.
Lim PO, Rodgers P, Cardale K, Watson AD, MacDonald TM . Potentially high prevalence of primary aldosteronism in a primary-care population. Lancet 1999; 353: 40.
Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, Ganzaroli C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, Mannelli M, Mattarello MJ, Moretti A, Palumbo G, Parenti G, Porteri E, Semplicini A, Rizzoni D, Rossi E, Boscaro M, Pessina AC, Mantero F PAPY Study Investigators. A prospective study of the prevalence of primary aldosteronism in 1125 hypertensive patients. J Am Coll Cardiol 2006; 48: 2293–2300.
Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP . Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990; 322: 1561–1566.
Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS, Vargiu P, Simongini I, Laragh JH . Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol 1992; 19: 1550–1558.
Mulè G, Nardi E, Guarino L, Cacciatore V, Geraci G, Calcaterra I, Oddo B, Vaccaro F, Cottone S . Plasma aldosterone and its relationship with left ventricular mass in hypertensive patients with early-stage chronic kidney disease. Hypertens Res 2015; 38: 276–283.
Schmieder RE, Langenfeld MR, Friedrich A, Schobel HP, Gatzka CD, Weihprecht H . Angiotensin II related to sodium excretion modulates left ventricular structure in human essential hypertension. Circulation 1996; 94: 1304–1309.
Vasan RS, Evans JC, Benjamin EJ, Levy D, Larson MG, Sundstrom J, Murabito JM, Sam F, Colucci WS, Wilson PW . Relations of serum aldosterone to cardiac structure: gender-related in the Framingham Heart Study. Hypertension 2004; 43: 957–962.
Rossi GP, Sacchetto A, Visentin P, Canali C, Graniero GR, Palatini P, Pessina AC . Changes in left ventricular anatomy and function in hypertension and primary aldosteronism. Hypertension 1996; 27: 1039–1045.
Muiesan ML, Salvetti M, Paini A, Agabiti-Rosei C, Monteduro C, Galbassini G, Belotti E, Aggiusti C, Rizzoni D, Castellano M, Agabiti-Rosei E . Inappropriate left ventricular mass in patients with primary aldosteronism. Hypertension 2008; 52: 529–534.
Goldkorn R, Yurenev A, Blumenfeld J, Fishman D, Devereux RB . Echocardiographic comparison of left ventricular structure and function in hypertensive patients with primary aldosteronism and essential hypertension. Am J Hypertens 2002; 15: 340–345.
Weber KT, Brilla CG . Pathologic hypertrophy and cardiac interstitium: fibrosis and renin-angiotensin-aldosterone system. Circulation 1991; 83: 1849–1865.
Brilla CG . Renin-angiotensin-aldosterone system and myocardial fibrosis. Cardiovasc Res 2000; 47: 1–3.
Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, Young WF Jr, Montori VM Endocrine Society. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2008; 93: 3266–3281.
Lin YH, Wang SM, Wu VC, Lee JK, Kuo CC, Yen RF, Liu KL, Huang KH, Chueh SC, Wang WJ, Lin LY, Chien KL, Ho YL, Chen MF, Wu KD TAIPAI study group. The association of serum potassium level with left ventricular mass in patients with primary aldosteronism. Eur J Clin Invest 2011; 41: 43–50.
Born-Frontsberg E, Reincke M, Rump LC, Hahner S, Diederich S, Lorenz R, Allolio B, Seufert J, Schirpenbach C, Beuschlein F, Bidlingmaier M, Endres S, Quinkler M Participants of the German Conn's Registry, Participants of the German Conn’s Registry. Cardiovascular and cerebrovascular comorbidities of hypokalemic and normokalemic primary aldosteronism: results of the German Conn’s Registry. J Clin Endocrinol Metab 2009; 94: 1125–1130.
Chen SX, Du YL, Zhang J, Gong YC, Hu YR, Chu SL, He QB, Song YY, Zhu DL . Aldosterone-to-renin ratio threshold for screening primary aldosteronism in Chinese hypertensive patients. Chinese J Cardiovasc Dis 2006; 34: 868–872.
Sahn DJ, DeMaria A, Kisslo J, Weyman A . The Committee on M-mode standardization of the American Society of echocardiography: recommendations regarding quantitation in M-mode echocardiography. Results of a survey of echocardiographic measurements. Circulation 1978; 58: 1072–1083.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU . Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270.
Muiesan ML, Salvetti M, Rizzoni D, Monteduro C, Castellano M, Agabiti-Rosei E . Persistence of left ventricular hypertrophy is a stronger predictor of cardiovascular events than baseline left ventricular mass or systolic performance: 10 years follow-up. J Hypertens 1996; 14 (suppl 5): S43–S49.
Teicholz LE, Kreulen T, Herman MV, Gorlin R . Problems in echocardiographic volume determination: echocardiographic-angiographic correlations in the presence or absence of asynergy. Am J Cardiol 1976; 37: 7–12.
Wallerson DC, Ganau A, Roman MJ, Devereux RB . Measurement of cardiac output by M-mode and two-dimensional echocardiography; application to patients with hypertension. Eur Heart J 1990; 11 (suppl 1): 67–78.
Devereux R, Koren M, de Simone G, Okin PM, Kligfield P . Methods for detection of left ventricular hypertrophy: application to hypertensive heart disease. Eur Heart J 1993; 14: 8–15.
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD . Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29: 277–314.
Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL . How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007; 28: 2539–2550.
Tsioufis C, Tsiachris D, Dimitriadis K, Stougiannos P, Missovoulos P, Kakkavas A, Stefanadis C, Kallikazaros I . Myocardial and aortic stiffening in the early course of primary aldosteronism. Clin Cardiol 2008; 31: 431–436.
Rodriguez L, Garcia M, Ares M, Griffin BP, Nakatani S, Thomas JD . Assessment of mitral annular dynamics during diastole by Doppler tissue imaging: comparison with mitral Doppler inflow in subjects without heart disease and in patients with left ventricular hypertrophy. Am Heart J 1996; 131: 982–987.
Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM . Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol 2005; 45: 87–92.
Srivastava TN, Young DB . Impairment of cardiac function by moderate potassium depletion. J Card Fail 1995; 1: 195–200.
Fitzovich DE, Hamaguchi M, Tull WB, Young DB . Chronic hypokalemia and the left ventricular responses to epinephrine and preload. J Am Coll Cardiol 1991; 18: 1105–1111.
Matsui H, Shimosawa T, Uetake Y, Wang H, Ogura S, Kaneko T, Liu J, Ando K, Fujita T . Protective effect of potassium against the hypertensive cardiac dysfunction. Hypertension 2006; 48: 225–231.
Wang Q, Domenighetti AA, Pedrazzini T, Burnier M . Potassium supplementation reduces cardiac and renal hypertrophy independent of blood pressure in DOCA/saltmice. Hypertension 2005; 46: 547–554.
Hattori T, Murase T, Sugiura Y, Nagasawa K, Takahashi K, Ohtake M, Ohtake M, Miyachi M, Murohara T, Nagata K . Effects of salt status and blockade of mineralocorticoid receptors on aldosterone-induced cardiac injury. Hypertens Res 2014; 37: 125–133.
Catena C, Colussi G, Lapenna R, Nadalini E, Chiuch A, Gianfagna P, Sechi LA . Long-term cardiac effects of adrenalectomy or mineralocorticoid antagonists in patients with primary aldosteronism. Hypertension 2007; 50: 911–918.
Schunkert H1, Hense HW, Muscholl M, Luchner A, Kürzinger S, Danser AH, Riegger GA . Associations between circulating components of the renin-angiotensin-aldosterone system and left ventricular mass. Heart 1997; 77: 24–31.
Kanno A, Kikuya M, Asayama K, Satoh M, Inoue R, Hosaka M, Metoki H, Obara T, Hoshi H, Totsune K, Sato T, Taguma Y, Sato H, Imai Y, Ohkubo T . Night-time blood pressure is associated with the development of chronic kidney disease in a general population: the Ohasama Study. J Hypertens 2013; 31: 2410–2417.
Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Bjorklund-Bodegård K, Richart T, Ohkubo T, Kuznetsova T, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Wang JG, Sandoya E, O’Brien E, Staessen JA on behalf of the International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes (IDACO) investigators. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007; 370: 1219–1229.
Gerdts E, Okin PM, de Simone G, Cramariuc D, Wachtell K, Boman K, Devereux RB . Gender differences in left ventricular structure and function during antihypertensive treatment: the Losartan intervention for endpoint reduction in hypertension study. Hypertension 2008; 51: 1109–1114.
Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA . Age- and gender-related ventricular vascular stiffening: a community-based study. Circulation 2005; 112: 2254–2262.
Weinberg EO, Thienelt CD, Katz SE, Bartunek J, Tajima M, Rohrbach S, Douglas PS, Lorell BH . Gender differences in molecular remodeling in pressure overload hypertrophy. J Am Coll Cardiol 1999; 34: 264–273.
Kararigas G, Dworatzek E, Petrov G, Summer H, Schulze TM, Baczko I, Knosalla C, Golz S, Hetzer R, Regitz-Zagrosek V . Sex-dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressureoverload. Eur J Heart Fail 2014; 16: 1160–1167.
Harvey BJ, Condliffe S, Doolan CM . Sex and salt hormones: rapid effects in epithelia. News Physiol Sci 2001; 16: 174–177.
Iacobellis G, Petramala L, Cotesta D, Pergolini M, Zinnamosca L, Cianci R, De Toma G, Sciomer S, Letizia C . Adipokines and cardiometablic profile in primary hyperaldosteronism. J Clin Endocrinol Metab 2010; 95: 2391–2398.
Rossi GP, Auchus RJ, Brown M, Lenders JW, Naruse M, Plouin PF, Satoh F, Young WF Jr . An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension 2014; 63: 151–160.
Mulatero P, Monticone S, Bertello C, Viola A, Tizzani D, Iannaccone A, Crudo V, Burrello J, Milan A, Rabbia F, Veglio F . Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab 2013; 98: 4826–4833.
Omura M, Sasano H, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T . Clinical characteristics of aldosterone-producing microadenoma, macroadenoma, and idiopathic hyperaldosteronism in 93 patients with primary aldosteronism. Hypertens Res 2006; 29: 883–889.
Satoh F, Abe T, Tanemoto M, Nakamura M, Abe M, Uruno A, Morimoto R, Sato A, Takase K, Ishidoya S, Arai Y, Suzuki T, Sasano H, Ishibashi T, Ito S . Localization of aldosterone-producing adrenocortical adenomas: significance of adrenal venous sampling. Hypertens Res 2007; 30: 1083–1095.
Acknowledgements
This work was supported by the state key program of the National Natural Science Foundation of China (81230071), the National High-tech R&D Program (2012AA02A516), the National Key Project for Basic Research (2011CB503905) and the National Science Foundation for Distinguished Young Scholars of China (81400179).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Hypertension Research website
Supplementary information
Rights and permissions
About this article
Cite this article
Yang, Y., Zhu, Lm., Xu, Jz. et al. Comparison of left ventricular structure and function in primary aldosteronism and essential hypertension by echocardiography. Hypertens Res 40, 243–250 (2017). https://doi.org/10.1038/hr.2016.127
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2016.127
This article is cited by
-
The differences of serum lipid profiles between primary aldosteronism and essential hypertension: a meta-analysis and systematic review
BMC Endocrine Disorders (2022)
-
Subclinical HMOD in Hypertension: Left Ventricular Diastolic Dysfunction
High Blood Pressure & Cardiovascular Prevention (2022)
-
Left ventricular remodeling and dysfunction in primary aldosteronism
Journal of Human Hypertension (2021)
-
A non-invasive left ventricular pressure-strain loop study on myocardial work in primary aldosteronism
Hypertension Research (2021)
-
Diurnal blood pressure pattern and cardiac damage in hypertensive patients with primary aldosteronism
Endocrine (2021)