Abstract
We investigated the independent and incremental role of worsening arterial stiffness in new-onset heart failure (HF) in patients with preclinical HF. We retrospectively studied 456 consecutive asymptomatic patients with HF risk factors (hypertension, obesity, type 2 diabetes mellitus, atrial fibrillation and ischemic heart disease) who underwent paired applanation tonometry examinations (median interval of 2.4 years) during 2006–2011. Brachial ankle pulse wave velocity (baPWV) was measured as a surrogate marker of arterial stiffness. Patients were followed up for admission for new-onset HF over a median duration of 4.9 years after the second examination. HF was observed in 30 patients (7%). The change in baPWV (∆baPWV) was significantly associated with hospitalization for new-onset HF, independent of and incremental to comorbidities, renal dysfunction, left ventricular (LV) dysfunction and baPWV at baseline. Even in patients with an LV ejection fraction of ⩾40%, ∆baPWV was significantly associated with hospitalization for new-onset HF after similar adjustments. When the patients were divided into groups based on this cutoff value of ⩾15% ∆baPWV and the generally accepted external cutoff value of ⩾1750 cm s−1 for baseline baPWV, the Kaplan–Meier estimates of the time of hospitalization for new-onset HF showed that a higher rate of HF was associated with higher baPWV at baseline and higher ∆baPWV (P=0.00005). In asymptomatic patients with cardiovascular risk factors, the deterioration in arterial stiffness was associated with hospitalization for new-onset HF, independent of and incremental with the clinical LV function and increased stiffness parameters at baseline.
Similar content being viewed by others
Introduction
Heart failure (HF) is a common, costly and potentially fatal condition.1 Increased arterial stiffness is an important risk factor for not only mortality but also the onset of HF in patients with cardiovascular diseases.2, 3, 4, 5 Most cardiovascular risk factors and aging cause increased arterial stiffness as a result of structural alterations, collagen deposition, reduced elastin content and fractions, and calcification via several biological processes, such as endothelial dysfunction, increased oxidative stress or another inflammatory injury.6 Further, enhanced arterial stiffness is associated with an increase in left ventricular (LV) afterload concurrent with hypertension, impaired coronary perfusion, increase in LV mass and LV diastolic dysfunction, which may contribute to the eventual onset of HF.7, 8, 9, 10
Arterial stiffness can improve after lifestyle modifications and the intake of antihypertensive drugs and statins.11, 12, 13, 14, 15 This improvement may be caused mainly by reduced blood pressure and improved endothelial function.11, 12 However, improvement in arterial stiffness is not necessarily observed in all patients irrespective of these interventions.14, 15 Guerin et al.15 reported that the absence of a pulse wave velocity decrease in response to a blood pressure decrease was a major factor contributing to the cardiovascular mortality of patients with end-stage renal failure. Considering the above, progression of arterial stiffness irrespective of appropriate intervention may maintain or worsen situations involving an increase in LV afterload and impaired coronary perfusion, which may lead to more LV dysfunction and facilitate the occurrence of HF. This is probably particularly important in patients with HF with preserved ejection fraction (EF) and in whom treatment has not yet been standardized.16 Brachial ankle pulse wave velocity (baPWV) is considered the standard method for measuring arterial stiffness because it has been established to be a simple, well-validated, reproducible technique of applanation tonometry.17 Therefore, we hypothesized that deterioration in arterial stiffness, determined by measurement of baPWV, is associated with new-onset HF. The aims of this study were to assess (1) the independent association and (2) incremental benefit of worsening baPWV for predicting the onset of HF relative to the general HF risks and baseline baPWV in asymptomatic patients with cardiovascular risk factors. Further, we investigated whether structural heart disease or the LV ejection fraction (LVEF) influences this association.
Methods
Study subjects
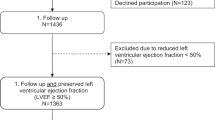
The process of patient selection is presented in Figure 1. In this retrospective cohort study, 642 consecutive adult patients (age range: 35–89 years) who underwent paired applanation tonometry examinations (⩾1 year interim between examinations) during April 2006–March 2011 at Kitaishikai Hospital were enrolled. Patients were excluded if they had an ankle-to-brachial blood pressure ratio of <0.90 (n=29) or if they did not have an echocardiographic examination and blood test within 7 days before and after the tonometry examinations (n=45). Through a chart review of the remaining patients, those without HF risk factors (hypertension, obesity, type 2 diabetes mellitus, atrial fibrillation and ischemic heart disease) (n=25) were excluded. Hypertension was defined as a systolic blood pressure of ⩾140 mm Hg, diastolic blood pressure of ⩾90 mm Hg, or current pharmacological treatment for hypertension. Obesity was defined as a body mass index of ⩾25 kg m−2.18 Type 2 diabetes mellitus was diagnosed according to standard criteria (plasma glucose level, either fasting or 2 h value in a 75 g oral glucose tolerance test or glycated hemoglobin A1c value). Atrial fibrillation was confirmed by a surface electrocardiogram. Ischemic heart disease was diagnosed if the patient had a history of myocardial infarction or coronary intervention or evident coronary artery disease based on cardiac catheterization. Further, patients with more than moderate valvular heart disease (n=45) were also excluded on the basis of their echo reports. In addition, five patients who had an evident symptom of HF at rest at the time of the first examination were excluded. Finally, 37 patients who were admitted owing to HF during the interim between the first and second examinations were excluded. Therefore, the final analysis was based on data from 456 asymptomatic patients without a history of admission owing to HF. The institutional review board of Kitaishikai Hospital approved the retrospective use of the patients’ data.
Pulse wave analysis
We measured baPWV as a surrogate marker of arterial stiffness. Subjects were examined in the morning (0830–0900) after a 12 h overnight fast in a quiet, temperature-controlled laboratory (22 °C) after 15 min of lying supine. The volume–plethysmographic method was used to noninvasively measure baPWV using previously validated equipment (form PWV/ABI; Colin Medical Technology, Komaki, Japan). A detailed description of this device has been reported previously.19 The average of the right and left baPWV values was used in the final analysis.
Echocardiography
Transthoracic echocardiography was performed by experienced sonographers using a commercially available ultrasound machine (Vivid 7; GE Vingmed, Horten, Norway). Conventional echocardiographic parameters were measured according to the recommendations of the American Society of Echocardiography.20, 21 LV mass was calculated according to the American Society of Echocardiography formula and was normalized to body surface area. LVEF was calculated by the method of disks using two-dimensional images. In the apical four-chamber view, the average of two early diastolic mitral annular tissue velocity (e′) values was also measured with the sample volume positioned at both the septal and lateral mitral annulus. The combined assessment of the transmitral early diastolic velocity (E) and e′ was used to calculate E/e′.
In the present study, we used the following criteria to determine the stage of HF, as discussed in previous reports.22, 23 Stage B HF was defined based on the presence of any of the following abnormalities: history of myocardial infarction, LV hypertrophy (LV mass index>95 g m−2 for women and >115 g m−2 for men), and E/e′>13. Stage A HF was defined as asymptomatic patients with HF risks without the structural and functional cardiac abnormalities described above.
Clinical data
Clinical parameters, including comorbidities and medical history, medication and serum markers, were collected via chart review. The average value of the right- and left-brachial blood pressure at the measurement of baPWV was used as the blood pressure value. The estimated glomerular filtration rate (eGFR) was calculated from serum creatinine using the revised equations for eGFR in Japan.24
Outcomes
Kitaishikai Hospital is a core hospital in Ozu City, Japan, that supports physicians, and most regular patients are normally admitted. Further, only Kitaishikai Hospital offers cardiology services in this medical district. Therefore, most patients with cardiovascular diseases, including HF, are referred to and admitted to Kitaishikai Hospital. So, only admission data for Kitaishikai Hospital were analyzed.
The primary outcome was admission for new-onset HF. The admission codes (ICD-10 (International Classification of Diseases, 10th revision) codes) used were I500 for congestive HF, I501 for LV failure and I509 for HF, unspecified. The secondary outcome was cardiovascular death or admission for new-onset HF. Cardiovascular death was defined as death due to acute myocardial infarction, sudden cardiac death, HF and stroke. These outcomes were followed up after the second examination. Outcomes were screened by the coded data and then confirmed by two cardiologists (TM and MS), blinded to the patients’ clinical data, reviewing each medical record or mailing or calling the patients, their relatives or the general practitioners. To increase the external validity of the primary outcome, the value of brain natriuretic peptide (BNP) at admission was checked in the patients with the primary outcome. Patients were censored at the time of the outcome or at the end of follow-up (31 July 2015).
Statistical analysis
Data are expressed as median (interquartile range (IQR)) or number (percentage). The percent change in the study parameters was calculated as the absolute difference divided by the absolute value at baseline. Overall, <5% of observations were missing, with the exception of e′ and E/e′ (6%). Missing e′ values were imputed from propensity models using parameters without missing data (age, sex, ischemic heart disease, diabetes mellitus, hypertension, atrial fibrillation, cerebrovascular disease, body mass index, systolic blood pressure, heart rate, LVEF, LV mass index and eGFR). E/e′ was calculated using the interpolated e′ value. Other continuous variables (<5%) were imputed using the corresponding mean value.
For multiple comparisons, one-way analysis of variance or χ2 tests were used, followed by Bonferroni correction as appropriate. Comparisons of the repeated values were carried out using a Wilcoxon signed-rank test. Univariate linear regression analyses were used to evaluate the associations between changes in baPWV (∆baPWV) and the study parameters. Univariate and multivariate Cox proportional hazards models were used to determine the contribution of the primary and secondary outcomes. The optimum model candidates were selected based on the significant (P<0.10) variables in the univariate analysis and clinically relevant parameters. The independence of ∆baPWV was examined using five different models. This was also confirmed in the subgroups of patients with LVEF>40%. The incremental value of ∆baPWV was also assessed using two Cox sequential multivariate models. Baseline baPWV was included in the model with ∆baPWV because their correlation was weak (R2=0.08). After the addition of ∆baPWV, the overall change in log-likelihood ratio χ2 was used to assess any increase in predictive power. Harrell’s C-statistic was used to evaluate model performance.25 The receiver-operating characteristic (ROC) curves were used to determine the optimal baPWV at baseline and ∆baPWV cutoff values for associations with the primary outcome. Survival was estimated using the Kaplan–Meier method, and differences in survival between groups were assessed using the log-rank test.
Statistical analysis was performed using standard statistical software packages (SPSS version 20.0; SPSS Inc., Chicago, IL, USA), and statistical significance was defined by P<0.05.
Results
Patient characteristics at baseline
Table 1 summarizes the clinical, therapeutic, echocardiographic and stiffness parameters. The median age at baseline was 71 years. The proportion of men was 309 (68%). Among the study population, 229 (50%) had ischemic heart disease; of these, 89 patients had a history of myocardial infarction. The median interval between the first and second tonometry examinations was 2.4 (IQR, 1.7–3.3) years. The median baPWV at baseline and follow-up and ∆baPWV was 1725 and 1734 cm s−1, respectively. The median ∆baPWV was 1.0% (IQR, −7 to –11%).
Outcomes
During a median follow-up of 4.9 (IQR 4.3–5.6) years after the second examination, 30 patients (7%) developed HF and 52 had cardiovascular deaths or admission for HF (11%). Of the 30 patients with the primary outcome, 17 (57%) were hospitalized owing to conditions that were categorized as clinical scenario 1.26 The most frequent precipitating factor for HF admission was ischemic heart disease (n=15, 50%), followed by arrhythmias (n=6, 20%), valvular heart disease (n=4, 13%), hypertension (n=3, 10%) and unknown (n=2, 7%). The BNP value at admission for HF in patients with the primary outcome (510 (IQR 281–1130) pg ml−1) was significantly higher than that at baseline (111 (IQR 61–184) pg ml−1, P<0.01).
Associations with ∆baPWV
Supplementary Table 1 compares the clinical characteristics in patients with and without baPWV progress (∆baPWV, ⩾0% vs. <0%). ∆baPWV was positively associated with ischemic heart disease and the change in blood pressure, and negatively associated with blood pressure at baseline, hypertension, calcium channel blockers use and e′.
Associations between baseline study parameters and hospitalization for new-onset HF
Supplementary Table 2 shows the univariate Cox regression analyses for the baseline study parameters that were associated with the primary outcome. The primary outcome was significantly associated with age, lower diastolic blood pressure, atrial fibrillation, higher BNP, lower eGFR, lower hemoglobin, larger left atrium, greater impairment of LV diastolic function, higher baPWV at baseline and follow-up, and deterioration of baPWV.
Independent association of ∆aPWV with the outcomes
In the multivariate Cox regression analyses (Table 2), age, atrial fibrillation, BNP, eGFR and E/e′ were significantly associated with hospitalization for new-onset HF. ∆baPWV was also independently associated with hospitalization for new-onset HF in all models. Similarly, ∆baPWV had an independent association with cardiovascular death or admission for HF (Supplementary Table 3).
Association of ∆aPWV and primary outcome in the subgroups
Of 436 patients with LVEF >40%, 28 were hospitalized for new-onset HF. In this subgroup, ∆baPWV was also independently associated with the primary outcome in the comorbidity, laboratory, echo, hemodynamic and medication models (Supplementary Table 4). Although ∆baPWV was not associated with the primary outcome in the demographic model, the effect size was similar. Stage B HF was identified in 259 patients (57%), 21 of whom were hospitalized for new-onset HF. The remaining patients (n=188, 43%) were considered to have stage A HF. In patients with stage B HF, ∆baPWV had a tendency to be associated with the primary outcome (per 10% increase, hazard ratio (HR=1.25 (95% confidence interval (CI) 0.97–1.61), P=0.09). In patients with stage A HF, ∆baPWV was significantly associated with the primary outcome (per 10% increase, HR=1.48 (95% CI 1.06–2.07)), P=0.02). In the ROC analysis, the optimal cutoff values of baPWV at baseline and ΔbaPWV for predicting the primary outcome in stage A were 1675 cm s−1 (area under the ROC curve (AUC)=0.66, sensitivity=79%, specificity=50%) and 11% (AUC=0.61, sensitivity=56%, specificity=78%), and those in stage B were 1870 cm s−1 (AUC=0.56, sensitivity=71%, specificity=60%) and 18% (AUC=0.66, sensitivity=38%, specificity=87%).
Incremental value of ∆aPWV
In the sequential Cox models, the model based on age, sex, mean blood pressure, heart rate, ischemic heart disease and atrial fibrillation demonstrated significant incremental improvements after the subsequent additions of BNP, eGFR, E/e′ and ∆baPWV (Figure 2). However, the model based on clinical parameters, BNP, eGFR and E/e′ was not significantly improved by adding baPWV at baseline (C-statistic 0.84–0.84, P=0.68). In addition, the model based on baPWV at baseline was significantly improved after the addition of ∆baPWV (C-statistic 0.65–0.72, P<0.01). In the final model, baPWV at baseline (per 100 cm s−1 increase, HR=1.21 (95% CI 1.11–1.33), P<0.01) and ∆baPWV (per 10% increase, HR=1.51 (95% CI 1.23–1.86), P<0.01) were independently associated with the primary outcome.
Incremental value of ∆baPWV over clinical and echocardiographic parameters as a correlate of hospitalization for new-onset heart failure. baPWV, brachial ankle pulse wave velocity; BNP, brain natriuretic peptide; CI, confidence interval; E, early diastolic velocity; e′, early diastolic mitral annular velocity; eGFR, estimated glomerular filtration rate; HR, hazard ratio.
Discriminative ability of ∆aPWV
The ROC curve analysis for the associations of hospitalization for new-onset HF (AUC=0.60, P=0.02) showed that a ∆baPWV of ⩾15% had optimal sensitivity (37%) and specificity (85%). When the patients were divided into groups based on this cutoff value of ⩾15% ∆baPWV and the generally accepted external cutoff value of ⩾1750 cm s−1 for baPWV,5 the Kaplan–Meier estimates of time to hospitalization for new-onset HF showed that a greater rate of HF (Figure 3a) and composite secondary outcome (Figure 3b) were associated with a higher baPWV at baseline and higher ∆baPWV.
Exploration of selection bias
Because of concern regarding referral bias in the selection process, we also evaluated the clinical characteristics of the patients who were admitted for HF during the interim between the first and second tonometry examinations (n=37) (Supplementary Table 5). Patients excluded because of HF admission before the second examination were significantly older and had a lower frequency of dyslipidemia, higher use of loop diuretics and spironolactone, higher BNP, and higher LV mass index than the enrolled patients who were admitted for HF after the second examination. baPWV at baseline and follow-up were significantly higher in patients with admission for HF than in patients not admitted for HF. Overall, LV dysfunction seemed to occur more frequently in the excluded patients. In addition, baPWV was likely to be increased in the patients who were admitted for HF before (P=0.09) and after the second examination (P=0.04). Consequently, ∆baPWV in both groups of patients admitted for HF was larger than that in patients not admitted for HF, but it was significant only in the patients admitted for HF after the second examination. The interval between the paired tonometry examinations was almost the same in all groups.
Discussion
This study on the prognostic significance of worsening arterial stiffness within a medium-term interval in asymptomatic patients with cardiovascular risk factors had several important findings. First, deterioration of arterial stiffness was independently associated with hospitalization for new-onset HF and with incremental changes in clinical and LV function parameters. Second, this association appeared to be applicable in the subgroup of patients with preserved LVEF. Finally, worsening arterial stiffness might provide an incremental benefit over increased arterial stiffness at baseline for the association with hospitalization for new-onset HF.
Possible mechanism of worsening arterial stiffness with HF development
Arterial stiffness has been reported to play a potential role in the pathophysiology of HF.7, 27 As the aorta stiffens, owing to several atherosclerotic risk factors, the velocity of the pressure wave increases and the reflected pressure wave eventually reaches the heart during systole rather than during diastole. This could be the cause of the augmented systolic blood pressure, the late peak of blood pressure (loading sequence: increase in late-systolic load) and the subsequent decreased diastolic blood pressure, all of which result in an increase in cardiac afterload and myocardial oxygen demand and impairment of myocardial blood flow.19, 28, 29, 30 In this setting, these processes could eventually influence LV diastolic dysfunction and lead to elevated LV filling pressure, congestion and HF.4, 5, 8
Recent literature has shown that arterial stiffness is treatable mainly by reducing blood pressure and improving endothelial function.11, 12, 13, 14, 15 In addition, no improvement in arterial stiffness irrespective of antihypertensive treatment has been shown to be associated with cardiovascular mortality in patients with advanced atherosclerosis, such as end-stage renal failure.15 Considering the points above, in cases in which arterial stiffness has not improved or has deteriorated, the hemodynamically harmful effect caused by increased arterial stiffness on LV function and coronary perfusion described above may continue, which may facilitate cardiovascular events, including the onset of HF. In the present study, worsening baPWV was significantly associated with deterioration in LV diastolic function, which may support this theory. In addition, the patients in the present study were elderly and had a high prevalence of ischemic heart disease. Therefore, coronary perfusion abnormalities may be more easily provoked. In fact, the most common cause of new-onset HF was ischemic heart disease.
A recent meta-analysis has shown that the strongest independent association for incident HF is ischemic heart disease.31 Arterial stiffness is closely related to endothelial function, which has an important role in the development of ischemic heart disease.27, 32 Therefore, the continuation of increased arterial stiffness may be closely associated with deterioration in endothelial function and, consequently, lead to deterioration in coronary perfusion, LV dysfunction and, eventually, the onset of HF. Otsuka et al.30 demonstrated the long-term harmful effects of decreased aortic distensibility on the heart in relation to coronary perfusion in dogs, which may corroborate this theory.
Finally, previous papers have shown that increased sympathetic tone was associated with increased pulse wave velocity.33 In addition, it is well known that sympathetic nerve activity is closely associated with the occurrence of HF.34 Possibly, elevated sympathetic tone may mediate the association between deteriorated arterial stiffness and HF progression. However, ∆baPWV may reflect a consequence of increased sympathetic tone in developing HF rather than a cause of HF.
Significance of the present study
In this study, the deterioration of baPWV demonstrated a significant incremental benefit over increased baPWV at baseline for the association with hospitalization for new-onset HF. Normally, the progression of arterial stiffness is slow.35 However, our results suggested that a combination of increased baseline arterial stiffness and a relatively rapid worsening of arterial stiffness over a medium-term follow-up might be more associated with adverse cardiac events, compared with the individual contribution of each parameter. These findings might highlight the importance of follow-up studies.
Currently, there is no best therapy for patients with HF with preserved EF, but several review articles have suggested that arterial stiffness modulates ventricular loading conditions and diastolic function, which are the key components of HF with preserved EF.16, 27 The present study also demonstrated the independent association between worsening baPWV and the onset of HF in patients with preclinical HF with preserved EF. In addition, the deterioration of baPWV seemed to be associated with the onset of HF even in the absence of cardiac structural and functional abnormalities. These findings suggest that deterioration of vascular function is an important contributor to the progression of HF even in the early stage of HF without advanced LV dysfunction. In general, advanced HF becomes intractable to treatment and leads to poor outcomes.36 Therefore, the present study results may indicate that early intervention for arterial stiffness is important for the prevention of HF.
In the present subgroup study, higher optimal cutoff values of ΔbaPWV and baseline baPWV in stage B than those in stage A were observed. These results might be explained by the higher age in stage B than that in stage A (73 (65–78) years vs 68 (59–74) years, P<0.01). Interestingly, the AUC of baseline ΔbaPWV for predicting new-onset HF was larger than that of the baseline baPWV in stage A, whereas the opposite result was observed in stage B. This result indicates that deterioration in arterial stiffness may contribute to the onset of HF in earlier stages of HF, whereas arterial stiffness itself has a stronger influence in later stages of HF. Therefore, the results suggest that attention should be given to deterioration in arterial stiffness, particularly in early-stage HF.
Similar to the findings in previous reports,14 the use of calcium channel blockers was significantly associated with a decrease in baPWV in our study. This effect may be mainly because calcium channel blockers enable the generation of stronger and more stable antihypertensive effects than those of other antihypertensive medications.37, 38 Although the use of calcium channel blockers was not directly associated with HF progression because the cause of HF was multifactorial,31 it might secondarily prevent HF development by improving baPWV.
However, the effect of ∆baPWV on the progress of HF might be smaller than that of the conventional risk factors, such as LV dysfunction and comorbidities. In fact, ∆baPWV was larger in excluded patients with higher HF risks who were hospitalized for HF before their follow-up examinations than in patients not admitted for HF, but the difference was not significant.
Study limitations
First, this retrospective analysis had limitations with respect to potential confounders and risk of bias, although conventional clinical risks were accounted for and the corresponding treatments were obtained. In addition, although we had paid the utmost attention to HF symptoms from the chart review, some patients with very mild HF symptoms may have been enrolled. Second, the interval between the examinations was medium-term, but it was not fixed. However, the deterioration of baPWV was significantly associated with hospitalization for new-onset HF after adjustment for the interval between examinations (per 10% increase, HR=1.31 (95% CI 1.06–1.61), P=0.01). In addition, the duration between examinations was comparable between the patients with and without hospitalization for new-onset HF. Finally, we included patients with atrial fibrillation in the present study. Although heart rate is an important confounder of pulse wave velocity assessment, previous articles have shown the prognostic significance of baPWV in patients with atrial fibrillation.39 Furthermore, echocardiographic parameters were determined if the ratio of preceding and pre-preceding intervals was almost 1, and then averaged from at least three cardiac cycles in these patients. In addition, ∆baPWV was significantly associated with incident HF even in the enrolled patients without atrial fibrillation (n=369, new-onset HF=20 per 10% increase, HR=1.35 (95% CI 1.05–1.74), P=0.02).
Clinical implication
The present study may imply the importance of paying attention to worsening arterial stiffness as one of the risk factors of overt HF, particularly in patients with preclinical HF. Currently, pulse wave velocity is readily available in the clinical setting.17 This makes it easier for worsening pulse wave velocity to be readily used in the clinical setting to identify patients at higher risk for admission for HF.
Conclusions
Deterioration of baPWV, which is an index of arterial stiffness, was associated with hospitalization for new-onset HF, independent of and incremental with clinical and echocardiographic parameters in the asymptomatic phase of HF, even in patients with preserved LVEF. Worsening arterial stiffness might have an incremental benefit over increased arterial stiffness for estimating HF progression. However, this was a single-center, retrospective cohort study. Larger multicenter studies are warranted to confirm these results.
References
Butler J, Fonarow GC, Gheorghiade M . Need for increased awareness and evidence-based therapies for patients hospitalized for heart failure. JAMA 2013; 310: 2035–2036.
Tsao CW, Lyass A, Larson MG, Levy D, Hamburg NM, Vita JA, Benjamin EJ, Mitchell GF, Vasan RS . Relation of central arterial stiffness to incident heart failure in the community. J Am Heart Assoc 2015; 4: 11.
Vlachopoulos C, Aznaouridis K, Stefanadis C . Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am College Cardiol 2010; 55: 1318–1327.
Chirinos JA, Kips JG, Jacobs DR Jr, Brumback L, Duprez DA, Kronmal R, Bluemke DA, Townsend RR, Vermeersch S, Segers P . Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis). J Am College Cardiol 2012; 60: 2170–2177.
Meguro T, Nagatomo Y, Nagae A, Seki C, Kondou N, Shibata M, Oda Y . Elevated arterial stiffness evaluated by brachial-ankle pulse wave velocity is deleterious for the prognosis of patients with heart failure. Circ J 2009; 73: 673–680.
Lakatta EG, Levy D . Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: The aging heart in health: links to heart disease. Circulation 2003; 107: 346–354.
Duprez DA . Arterial stiffness/elasticity in the contribution to progression of heart failure. Heart Fail Clin 2012; 8: 135–141.
Mottram PM, Haluska BA, Leano R, Carlier S, Case C, Marwick TH . Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart 2005; 91: 1551–1556.
Schillaci G, Battista F, Settimi L, Anastasio F, Pucci G . Cardio-ankle vascular index and subclinical heart disease. Hypertens Res 2015; 38: 68–73.
Sibiya MJ, Norton GR, Hodson B, Redelinghuys M, Maseko MJ, Majane OH, Libhaber E, Woodiwiss AJ . Gender-specific contribution of aortic augmentation index to variations in left ventricular mass index in a community sample of African ancestry. Hypertens Res 2014; 37: 1021–1027.
Petersen KS, Blanch N, Keogh JB, Clifton PM . Effect of weight loss on pulse wave velocity: systematic review and meta-analysis. Arterioscl ThrombVasc Biol 2015; 35: 243–252.
Peng F, Pan H, Wang B, Lin J, Niu W . The impact of angiotensin receptor blockers on arterial stiffness: a meta-analysis. Hypertension Res 2015; 38: 613–620.
Sahebkar A, Pecin I, Tedeschi-Reiner E, Derosa G, Maffioli P, Reiner Z . Effects of statin therapy on augmentation index as a measure of arterial stiffness: a systematic review and meta-analysis. Int J Cardiol 2016; 212: 160–168.
Ichihara A, Hayashi M, Koura Y, Tada Y, Hirota N, Saruta T . Long-term effects of intensive blood-pressure lowering on arterial wall stiffness in hypertensive patients. Am J Hypertens 2003; 16 (11 Pt 1): 959–965.
Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM . Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation 2001; 103: 987–992.
Senni M, Paulus WJ, Gavazzi A, Fraser AG, Díez J, Solomon SD, Smiseth OA, Guazzi M, Lam CS, Maggioni AP, Tschöpe C, Metra M, Hummel SL, Edelmann F, Ambrosio G, Stewart Coats AJ, Filippatos GS, Gheorghiade M, Anker SD, Levy D, Pfeffer MA, Stough WG, Pieske BM . New strategies for heart failure with preserved ejection fraction: the importance of targeted therapies for heart failure phenotypes. Eur Heart J 2014; 35: 2797–2815.
Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y . Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res 2002; 25: 359–364.
The Examination Committee of Criteria for 'Obesity Disease' in Japan, Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circ J 2002; 66: 987–992.
Saito M, Okayama H, Nishimura K, Ogimoto A, Ohtsuka T, Inoue K, Hiasa G, Sumimoto T, Higaki J . Possible link between large artery stiffness and coronary flow velocity reserve. Heart 2008; 94: e20.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU . Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39 e14.
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD . Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29: 277–314.
Kosmala W, Jellis CL, Marwick TH . Exercise limitation associated with asymptomatic left ventricular impairment: analogy with stage B heart failure. J Am College Cardiol 2015; 65: 257–266.
Ammar KA, Jacobsen SJ, Mahoney DW, Kors JA, Redfield MM, Burnett JC Jr, Rodeheffer RJ . Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation 2007; 115: 1563–1570.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A . Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992.
Harrell FE Jr, Lee KL, Mark DB . Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996; 15: 361–387.
Mebazaa A, Gheorghiade M, Pina IL, Harjola VP, Hollenberg SM, Follath F, Rhodes A, Plaisance P, Roland E, Nieminen M, Komajda M, Parkhomenko A, Masip J, Zannad F, Filippatos G . Practical recommendations for prehospital and early in-hospital management of patients presenting with acute heart failure syndromes. Crit Care Med 2008; 36 (1 Suppl): S129–S139.
Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J . Endothelial dysfunction, arterial stiffness, and heart failure. J Am College Cardiol 2012; 60: 1455–1469.
Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH . Aortic stiffness: current understanding and future directions. J Am College Cardiol 2011; 57: 1511–1522.
Hori M, Inoue M, Kitakaze M, Tsujioka K, Ishida Y, Fukunami M, Nakajima S, Kitabatake A, Abe H . Loading sequence is a major determinant of afterload-dependent relaxation in intact canine heart. Am J Physiol 1985; 249 (4 Pt 2): H747–H754.
Ohtsuka S, Kakihana M, Watanabe H, Sugishita Y . Chronically decreased aortic distensibility causes deterioration of coronary perfusion during increased left ventricular contraction. J Am College Cardiol 1994; 24: 1406–1414.
Yang H, Negishi K, Otahal P, Marwick TH . Clinical prediction of incident heart failure risk: a systematic review and meta-analysis. Open Heart 2015; 2: e000222.
Kinlay S, Libby P, Ganz P . Endothelial function and coronary artery disease. Curr Opin Lipidol 2001; 12: 383–389.
Nakao M, Nomura K, Karita K, Nishikitani M, Yano E . Relationship between brachial-ankle pulse wave velocity and heart rate variability in young Japanese men. Hypertens Res 2004; 27: 925–931.
Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J . The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am College Cardiol 2009; 54: 1747–1762.
Tomiyama H, Arai T, Koji Y, Yambe M, Motobe K, Zaydun G, Yamamoto Y, Hori S, Yamashina A . The age-related increase in arterial stiffness is augmented in phases according to the severity of hypertension. Hypertens Res 2004; 27: 465–470.
Hunt SA . ACC/AHA guidelines: A-, B-, C-, and D-based approach to chronic heart failure therapy. Eur Heart J Suppl 2006; 8 (Suppl E): E3–E5.
Leenen FH, Nwachuku CE, Black HR, Cushman WC, Davis BR, Simpson LM, Alderman MH, Atlas SA, Basile JN, Cuyjet AB, Dart R, Felicetta JV, Grimm RH, Haywood LJ, Jafri SZ, Proschan MA, Thadani U, Whelton PK, Wright JT Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group. Clinical events in high-risk hypertensive patients randomly assigned to calcium channel blocker vs. angiotensin-converting enzyme inhibitor in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Hypertension 2006; 48: 374–384.
Webb AJ, Fischer U, Mehta Z, Rothwell PM . Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet 2010; 375: 906–915.
Chen SC, Lee WH, Hsu PC, Lin MY, Lee CS, Lin TH, Voon WC, Lai WT, Sheu SH, Su HM . Association of brachial-ankle pulse wave velocity with cardiovascular events in atrial fibrillation. Am J Hypertens 2015; 29: 348–356.
Acknowledgements
The authors gratefully acknowledge the assistance of Mr Masaru Morino in the collection of outcome data. The study was approved by the institutional review board of Kitaishikai Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Hypertension Research website
Rights and permissions
About this article
Cite this article
Aisu, H., Saito, M., Inaba, S. et al. Association of worsening arterial stiffness with incident heart failure in asymptomatic patients with cardiovascular risk factors. Hypertens Res 40, 173–180 (2017). https://doi.org/10.1038/hr.2016.116
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2016.116
Keywords
This article is cited by
-
Association of TyG index and TG/HDL-C ratio with arterial stiffness progression in a non-normotensive population
Cardiovascular Diabetology (2021)
-
Effect of tofogliflozin on arterial stiffness in patients with type 2 diabetes: prespecified sub-analysis of the prospective, randomized, open-label, parallel-group comparative UTOPIA trial
Cardiovascular Diabetology (2021)
-
Determinants of pulse pressure amplification in hypertensive and diabetic patients
Hypertension Research (2019)
-
Longitudinal interaction between APOA5 -1131T>C and overweight in the acceleration of age-related increase in arterial stiffness through the regulation of circulating triglycerides
Hypertension Research (2019)
-
Healthy aging and carotid performance: strain measures and β-stiffness index
Hypertension Research (2018)