Abstract
Myocarditis is a clinically severe disease; however, no effective treatment has been established. The aim of this study was to determine whether cacao bean (Theobroma cacao) polyphenols ameliorate autoimmune myocarditis. We used an experimental autoimmune myocarditis (EAM) model in Balb/c mice. Mice with induced EAM were treated with a cacao polyphenol extract (CPE, n=12) or vehicle (n=12). On day 21, hearts were harvested and analyzed. Elevated heart weight to body weight and fibrotic area ratios as well as high cardiac cell infiltration were observed in the vehicle-treated EAM mice. However, these increases were significantly suppressed in the CPE-treated mice. Reverse transcriptase-PCR revealed that mRNA expressions of interleukin (Il)-1β, Il-6, E-selectin, vascular cell adhesion molecule-1 and collagen type 1 were lower in the CPE group compared with the vehicle group. The mRNA expressions of nicotinamide adenine dinucleotide phosphate-oxidase (Nox)2 and Nox4 were increased in the vehicle-treated EAM hearts, although CPE treatment did not significantly suppress the transcription levels. However, compared with vehicle treatment of EAM hearts, CPE treatment significantly suppressed hydrogen peroxide concentrations. Cardiac myeloperoxidase activity, the intensity of dihydroethidium staining and the phosphorylation of nuclear factor-κB p65 were also lower in the CPE group compared with the vehicle group. Our data suggest that CPE ameliorates EAM in mice. CPE is a promising dietary supplement to suppress cardiovascular inflammation and oxidative stress.
Similar content being viewed by others
Introduction
Acute myocarditis causes acute heart failure as well as dilated cardiomyopathy.1 Patients with the severest form of myocarditis may suffer from rapidly progressing heart failure, shock or arrhythmia.2, 3 Although there are many approaches to the treatment of this disease, effective treatments have not been fully established. Murine experimental autoimmune myocarditis (EAM), which is induced by immunizing mice with myocardial self-antigens, resembles the autoimmunological process of acute myocarditis.4, 5, 6 This model has been used to study the pathogenesis of clinical myocarditis.
Plant polyphenols are known to suppress inflammation, oxidative stress and fibrosis.6, 7 Cacao beans (Theobroma cacao), which are the source of chocolate, are rich in polyphenols such as (−)-epicatechin and their oligomeric procyanidins.8 Pérez-Jiménez and co-authors9 surveyed the 100 richest dietary sources of polyphenols (mg per 100 g or mg per 100 ml) out of 452 foods and have reported that cocoa powder is in 4th place (ahead of blackberry, 30th; apple, 48th; and red wine, 53rd). Cacao polyphenols enhance antioxidative activity9, 10 and downregulate T-lymphocyte activation.11 A recent study has reported that cacao polyphenols inhibit the development of atherosclerosis in apolipoprotein E-deficient mice.12 Another study has shown that regular consumption of dark chocolate reduces myocardial inflammation in mice exposed to air pollution.13 However, no study revealing the effects of cacao polyphenols on autoimmune myocarditis has been reported.
Several studies have demonstrated that adiponectin elicits an anti-inflammatory response14 and plant polyphenols, including procyanidins, induce adiponectin gene expression.15, 16 In additiona, a recent review has suggested that cocoa polyphenols may have the potential to enhance adiponectin gene expression;17 however, concrete data were not shown.
The purpose of this study was to determine whether cacao polyphenols ameliorate autoimmune myocarditis. We provide the first demonstration that cacao polyphenols ameliorate EAM, indicating that cacao polyphenols are a promising dietary supplement to suppress cardiovascular inflammation and oxidative stress.
Methods
Experimental autoimmune myocarditis
Six-week-old male Balb/c mice were obtained from CREA Japan, (Tokyo, Japan). They were fed a standard diet and were maintained in compliance with the animal welfare guidelines of the Institute of Experimental Animals, Tokyo Medical and Dental University. This study was approved by the Animal Care and Use Committee of Tokyo Medical and Dental University. Purified synthetic myosin peptide,4 Myhc-α614-629 (Japan Bio Services, Saitama, Japan) was emulsified with an equal volume of complete Freund's adjuvant supplemented with Mycobacterium tuberculosis H37RA (Difco, Sparks, MD, USA). On days 0 and 7, the mice were injected in the back subcutaneously with 0.2 ml of the emulsion, yielding an immunizing dose of 150 μg of synthetic myosin peptide per mouse.
Cacao polyphenol administration
We used cacao polyphenol extract powder (CPE: Meiji, Tokyo, Japan), which contained ~80% cacao polyphenols, in this study. This extract contained (−)-epicatechin (16.6%), procyanidin B2 (9.4%), procyanidin C1 (5.1%), cinnnamtannin A2 (2.3%), procyanidin B5 (1.3%), (+)-catechin (0.8%) and other polyphenolic compounds. The immunized mice were randomly assigned into two groups as follows: the CPE group (n=12) received CPE in distilled water (1.2 mg kg−1 per day), and the vehicle group (distilled water only, n=12) received distilled water. All treatments were administered orally from day 0 to day 21. The control mice were not immunized and received vehicle in the same manner (n=4).
Blood pressure
Systolic, mean and diastolic blood pressure was measured using a tail-cuff system (BP-98A, Softron, Tokyo, Japan) weekly between 10:00 am and 12:00 pm. Unanesthetized, awake mice were prewarmed for 10 min at 37 °C in a thermostatically controlled heating cabinet. An average of five recordings was collected for each individual value.
Echocardiogram
Transthoracic echocardiography was performed according to a previously described method.18 The animals were anesthetized by i.p. administration (0.1 ml per 10 g body weight) of 3.6% chloral hydrate (2,2,2-trichloro-1,1-ethanediol, Wako Pure Chemical Industries, Osaka, Japan) in saline on day 21. An echocardiographic machine with a 14 MHz transducer (Toshiba, Tokyo, Japan) was used for left ventricular echocardiographic recording. A two-dimensional targeted M-mode and B-mode echocardiogram was obtained along the short-axis view of the left ventricle at the level of the papillary muscles. The left ventricular internal dimension diastolic, left ventricular internal dimension systolic, ejection fraction and fractional shortening were calculated from the M-mode echocardiograms.19 To analyze cardiac function, two investigators independently measured the contraction, and the values were averaged.
Histopathology
Hearts were harvested immediately after the mice were killed on day 21. We obtained a mid-ventricular section, and slices were stained with hematoxylin and eosin and Mallory’s trichrome (Mallory). The ratio of the area of cell infiltration was calculated according to a previously described method.20 The area of fibrosis and necrotic changes were calculated as fibrotic area by using a modification of the method used to calculate the ratio of the area of cell infiltration. In brief, the cross-sections of Mallory-stained heart were photographed, and the photographs were printed onto paper. The total area of the myocardium and the fibrotic areas (consisting of connective tissue) were accurately outlined on the paper by microscopically examining the original Mallory-stained cross-sections. The papers were then scanned. The percentage area of the fibrotic myocardium was determined via computer analysis using the public domain NIH Image program (Image J: National Institutes of Health, USA).
Immunohistochemistry
Immunohistochemistry was performed to examine CD4 (#550296, BD Biosciences, Tokyo, Japan) and Gr-1 (#108401, BioLegend, San Diego, CA, USA) expression in the hearts on day 21. The sections were incubated with unlabeled primary antibodies overnight at 4 °C, washed in phosphate-buffered saline, and then incubated with secondary antibodies (Histofine; Nichirei, Tokyo, Japan). The sections were washed in phosphate-buffered saline and incubated with an aminoethylcarbazolecomplex (Nichirei). CD4- and Gr-1-positive cells per high-power field were counted in six randomly selected fields, and the counts were averaged.
RNA extraction and real-time PCR
Total RNA was extracted using TRIsure (Bioline, Tokyo, Japan) according to the manufacturer’s protocol. Complementary DNA was prepared with a reverse transcriptase-PCR kit (Life Technologies Japan, Tokyo, Japan). PCR was performed with the PCR kit in the presence of predesigned oligo primers for interleukin (Il)-1β (Il1b, Mm00434228_m1), Il-6 (Il-6, Mm00446190_m1), Il-10 (Il-10, Mm00439616_m1), tumor necrosis factor-α (Tnf, Mm00443260_g1), E-selectin (Sele, Mm01310197_m1), vascular cell adhesion molecule-1 (Vcam, Mm01320970), intercellular adhesion molecule-1 (Icam, Mm00516023), collagen type I (Col1a1, Mm00801666_g1), nicotinamide adenine dinucleotide phosphate-oxidase (Nox)2 (Mm01287743_m1) and Nox4 (Mm00479245_m1). The mRNA levels were quantified and normalized to the levels of 18 s (4319413E). The complementary DNA was run in duplicate, and quantitative data were obtained using the comparative Ct (ΔΔCt) method.21 To determine the mRNA expression of adiponectin, Power SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA) was used for the real-time PCR reaction. The sequences of the adiponectin primers were described previously,22 and glyceraldehyde 3-phosphate dehydrogenase23 was used as an internal control.
Serum concentrations of anticardiac myosin titers
Measurements of anticardiac myosin titers in serum were performed as previously described,24 with some modifications. Microplates (96 wells) were coated with 100 μl per well of Myhc-α614-629 (10 μg ml−1) in bicarbonate buffer (Polysciences, Warrington, PA, USA) and left overnight. Mouse IgG secondary antibodies (NA931: GE Healthcare, Tokyo, Japan), diluted 1:1000, were used for detection. Serum samples were diluted 1:100, 1:200, 1:400, 1:800, 1:1600, 1:3200 and 1:6400. Tetramethylbenzidine substrate (T0440, Sigma-Aldrich Japan, Tokyo, Japan) was added to each well, and the plates were allowed to stand for 30 min. The reaction was stopped by adding 0.5 m H2SO4. Optical densities were determined at 450 nm. End point antibody titers for each individual mouse were calculated as the greatest positive dilution of antibody.
Hydrogen peroxide concentration and MPO activity in the heart
Relative concentrations of cardiac H2O2 were determined using a kit (ADI-907-015, Enzo Life Science, Farmingdale, NY, USA). Equal volumes of heart tissues were homogenized in 50 mm phosphate (pH 6.0) and centrifuged. The supernatant was used as the sample. The optical density was detected at 550 nm. Myeloperoxidase (MPO) activity in the hearts was assessed using an adaptation of a previously described protocol.25 One unit of MPO activity was defined as a change in A460 of 1.0 after 2 min, and the results were expressed as mU of MPO activity per mg of heart tissue (mU mg−1).
DHE staining
The cardiac O2− level was evaluated by dihydroethidium (DHE) staining using an adaptation of a previously described protocol.26, 27 Ethidium fluorescence was detected by fluorescence microscopy.
Western blotting
Nuclear factor-κ B (NF-κB) activity in the heart was assessed using an adaptation of a previously described protocol.18
Statistical analysis
Statistical analysis was performed using the SPSS Base System 14.0 J for Windows (IBM Japan, Tokyo, Japan). Values are reported as the mean±s.e.m. Group comparisons were made by unpaired t-test, Kruskal–Wallis test or one-way analysis of variance followed by Tukey’s comparison tests. Differences were considered statistically significant at P<0.05.
Results
Blood pressure and echocardiogram
There were no significant differences in blood pressure or heart rate (Figures 1a-d). In addition, the echocardiogram data did not significantly differ between the CPE group and the vehicle group.
CPE suppressed cardiac remodeling
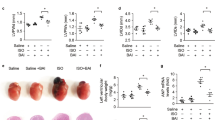
Heart weight to body weight ratios increased in the vehicle group but did not increase in the CPE group (Figure 1e). Histological sections of EAM hearts were stained with hematoxylin and eosin and Mallory. Cardiac myosin sensitization increased cell infiltration and the fibrotic area ratio in the vehicle-treated hearts. However, CPE treatment suppressed these exacerbations (Figure 2).
CPE suppresses cell infiltration and fibrosis. (a) Representative hematoxylin and eosin (HE) and Mallory staining of heart sections from control mice and from vehicle- and CPE-treated mice on day 21. Bar, 1 mm (× 20) and 50 μm (× 400). Cardiac (b) cell infiltration and (c) fibrotic area ratio. Control group, n=3; Vehicle group, n=10; CPE group, n=11. Values are mean±s.e.m. *P<0.05 vs control. †P<0.05 vs vehicle. A full color version of this figure is available at Hypertension Research online.
Immunohistochemistry
Immunohistochemical analysis indicated that there was a significant difference in the number of Gr-1-positive cells per entire area (mm2) between the CPE group and the vehicle group (Figures 3a and b). Similarly, there was a significant difference in the number of CD4-positive cells per entire area (mm2) between the CPE group and the vehicle group (Figures 3a and c).
(a) Representative Gr-1 (× 50; bar, 500 μm) and CD4 (× 100; bar, 200 μm) immunohistochemistry of the heart. (b and c) The numbers of immunohistochemically stained cells were counted in six random fields per section and averaged (control group, n=3; Vehicle group, n=6; CPE group, n=6). Values are mean±s.e.m. *P<0.05 vs control. †P<0.05 vs vehicle. A full color version of this figure is available at Hypertension Research online.
CPE inhibited the gene expression of pro-inflammatory cytokines, adhesion molecules and collagen but not of superoxide-generating enzymes
We measured gene expression in the hearts and found that Il-1β and Il-6 mRNA expression was lower in the CPE group than in the vehicle group (Figures 4a and b). However, there were no significant differences in Il-10 or Tnf mRNA expression levels between the CPE and vehicle group (Figures 4c and d). Gene expression of the cell infiltration-related adhesion molecules E-selectin and vascular cell adhesion molecule-1, but not intercellular adhesion molecule-1, was inhibited by CPE in the cardiac myosin-sensitized hearts (Figures 4e–g). Moreover, collagen type 1 mRNA expression was lower in the CPE group compared with the vehicle group (Figure 4h). Gene expressions of the superoxide-generating enzymes Nox2 and Nox4 were upregulated after EAM; however, CPE treatment did not significantly suppress the transcription levels (Figures 4i and j).
mRNA expression in hearts on day 21. (a) Il1b, (b) Il6, (c) Il-10, (d) TNF, (e) Sele, (f) Vcam, (g) Icam, (h) Col1a1, (i) Nox2, and (j) Nox4 mRNA expression levels. The ratio was calculated relative to 18 s expression as a control. Control group, n=3; vehicle group, n=7; CPE group, n=7. Values are mean±s.e.m. *P<0.05 vs control. †P<0.05 vs vehicle.
CPE treatment did not affect the autoimmune response or adiponectin expression
Serum samples from EAM mice showed increased autoantibodies, but there were no significant differences between the CPE- and vehicle-treated groups (Figure 5a). We examined whether CPE treatment altered adiponectin gene expression in heart and visceral white adipose tissue. Although the hearts did not express adiponectin mRNA, white adipose tissue enhanced the mRNA levels. CPE treatment did not change the adiponectin mRNA level (Figure 5b).
CPE did not affect the autoimmune response or adiponectin expression. (a) Anticardiac myosin titers in serum collected 21 days after immunization, as measured by enzyme-linked immunosorbent assay (ELISA). Control group, n=3; vehicle group, n=6; CPE group, n=6. (b) Adiponectin mRNA expression in hearts and white adipose tissue (WAT). mRNA levels were normalized to GAPDH. n=4 per group.
CPE treatment inhibited oxidative stress and NF-κB activation
Cardiac hydrogen peroxide concentrations were increased in the vehicle group; however, CPE treatment significantly suppressed these levels (Figure 6a). We also measured MPO activity and DHE staining. Cardiac MPO activity was increased in the vehicle-treated hearts. In contrast, MPO activity was not increased in the CPE-treated hearts (Figure 6b). Quantitative analysis showed that the intensity of DHE staining was significantly greater in the vehicle group. However, CPE treatment reduced the intensity of DHE staining (Figure 6c). Finally, we evaluated NF-κB activation by EAM induction. CPE treatment, compared with vehicle treatment, suppressed the levels of phosphorylated NF-κB p65 (Figure 6d).
(a) Cardiac hydrogen peroxide concentration on day 21. Control group, n=3; vehicle group, n=5; CPE group, n=5. (b) Cardiac MPO activity on day 21. Control group, n=3; vehicle group, n=6; CPE group, n=6. (c) Fluorescence detection of superoxide in hearts on day 21. Representative superoxide detection in cross-sections of heart is shown via DHE staining. Magnification, × 400. n=3 per group. (d) Activity of NF-κB in hearts on day 21. Representative western blots demonstrating the expression of phosphorylated (phos)-NF-κB p65 (Ser536) and total-NF-κB p65. All values are mean±s.e.m. *P<0.05 vs control. †P<0.05 vs vehicle. A full color version of this figure is available at Hypertension Research online.
Discussion
Our results revealed that CPE suppressed cardiac remodeling, inflammation and oxidative stress in mice immunized with cardiac myosin. Although CPE treatment did not significantly suppress Nox2 and Nox4 transcription levels, compared with vehicle treatment of EAM hearts, CPE treatment significantly suppressed hydrogen peroxide concentrations. Nox2 and Nox4 generate superoxide, which is easily converted into hydrogen peroxide.28 Serum antibody titers for cardiac myosin, which is an indicator of autoimmune disease, were not inhibited by CPE treatment. Moreover, although several studies have demonstrated that adiponectin elicits an anti-inflammatory response14 and that plant polyphenols, including procyanidins, induce adiponectin gene expression,15, 16 CPE administration did not alter adiponectin mRNA levels in this study. A recent review has suggested that cocoa polyphenols affect adiponectin gene expression;17 however, concrete data were not shown. It has been suggested that the pathological process of EAM involves the following: antigen presentation of cardiac myosin to T cells by macrophages/dendritic cells; antibody production by B cells; accumulation and infiltration by CD4 T cells and neutrophils; enhancement of inflammation and oxidative stress; and fibrosis.29 Therefore, we propose that the mechanism by which CPE inhibited the EAM in our study may involve the targeting of inflammation and oxidative stress rather than the early immunological processes of macrophages/dendritic cells and the production of autoantibodies by B cells. Thus, this study reveals that CPE ameliorates myocarditis without inhibiting the early stages of the immunological response. The EAM hearts showed cell infiltration; in particular, infiltration of CD4-positive T cells and neutrophils was observed. CD4-positive T cells have a major role in inflammation in EAM.30, 31 Therefore, we suggest that the antioxidative inhibition of CD4-positive T cells was the main pathophysiological mechanism by which the administration of CPE ameliorated heart inflammation.
We have previously reported that green tea polyphenols, which include epigallocatechin gallate as a principal component, inhibits EAM in rodents.6 Because cacao polyphenols include epicatechin and oligomeric procyanidins as major components, they may contribute to the EAM inhibition. However, no reports have demonstrated the pathophysiological relationship between myocarditis and these polyphenols. Some studies have shown that epicatechin inhibits the lipopolysaccharide-induced increase of pro-inflammatory cytokines in vitro32 and that i.p. injection of procyanidins ameliorates experimental autoimmune encephalomyelitis.33 In this study, we fed CPE to mice and found that the absorption ratio of epicatechin was ~30–50%;10 in contrast, the ratio of procyanidins was <1%.34 A previous study has shown that procyanidins enhance the production of adiponectin.16 However, we could not detect a CPE-mediated increase in adiponectin mRNA in either the hearts or white adipose tissue of the EAM mice. Therefore, epicatechins may have had a main role in EAM inhibition in this study. Further experiments should be performed to clarify the effects of epicatechins and procyanidins on EAM.
Pro-inflammatory cytokines, oxidative stress and inflammation affect the pathological condition of EAM.29 In particular, NF-κB is a central activator of inflammation and immune development.18, 35 NF-κB also activates cell proliferation, apoptosis and the expression of adhesion molecules.35 Previously, we have reported strong expression of NF-κB p65 in the nuclei of infiltrating cells in EAM hearts.18 Other studies have revealed that NF-κB transactivates Il-1β mRNA expression36 and that Il-1β signaling is critical for the development of EAM.37 Thus, CPE can inhibit EAM though NF-κB/Il-1β suppression. We suspect that CPE may inhibit Il-1β, Il-6 and NF-κB activation via its antioxidant function. Oxidative stress and NF-κB/Il-1β are known to form a positive autoregulatory loop.36, 38 CPE may inhibit this loop via an antioxidative effect. However, although Tnf is an important inflammatory factor in the pathogenesis of EAM,39, 40 we found that CPE did not inhibit Tnf mRNA expression in the EAM mice. Because Tnf induces the production of reactive oxygen species,41 we suggest that CPE ameliorates EAM by via an antioxidant effect.
Recently, supplements containing polyphenols from green tea, grapes, and coffee beans have gained attention for disease prevention; however, these effects have yet to be fully validated. In the future, supplements containing CPE may be useful to treat or prevent various cardiovascular diseases and other autoimmune diseases. In conclusion, CPE is a promising dietary supplement that may lead to the suppression of cardiovascular inflammation and oxidative stress.
References
Dennert R, Crijns HJ, Heymans S . Acute viral myocarditis. Eur Heart J 2008; 29: 2073–2082.
Haas GJ . Etiology, evaluation, and management of acute myocarditis. Cardiol Rev 2001; 9: 88–95.
Liu PP, Mason JW . Advances in the understanding of myocarditis. Circulation 2001; 104: 1076–1082.
Eriksson U, Kurrer MO, Schmitz N, Marsch SC, Fontana A, Eugster HP, Kopf M . Interleukin-6-deficient mice resist development of autoimmune myocarditis associated with impaired upregulation of complement C3. Circulation 2003; 107: 320–325.
Wu L, Ong S, Talor MV, Barin JG, Baldeviano GC, Kass DA, Bedja D, Zhang H, Sheikh A, Margolick JB, Iwakura Y, Rose NR, Cihakova D . Cardiac fibroblasts mediate IL-17A-driven inflammatory dilated cardiomyopathy. J Exp Med 2014; 211: 1449–1464.
Suzuki J, Ogawa M, Futamatsu H, Kosuge H, Sagesaka YM, Isobe M . Tea catechins improve left ventricular dysfunction, suppress myocardial inflammation and fibrosis, and alter cytokine expression in rat autoimmune myocarditis. Eur J Heart Fail 2007; 9: 152–159.
Ginter E, Simko V . Plant polyphenols in prevention of heart disease. Bratisl Lek Listy 2012; 113: 476–480.
Natsume M, Osakabe N, Yamagishi M, Takizawa T, Nakamura T, Miyatake H, Hatano T, Yoshida T . Analyses of polyphenols in cacao liquor, cocoa, and chocolate by normal-phase and reversed-phase HPLC. Biosci Biotechnol Biochem 2000; 64: 2581–2587.
Perez-Jimenez J, Neveu V, Vos F, Scalbert A . Identification of the 100 richest dietary sources of polyphenols: an application of the Phenol-Explorer database. Eur J Clin Nutr 2010; 64 (Suppl 3): S112–S120.
Baba S, Osakabe N, Natsume M, Muto Y, Takizawa T, Terao J . Absorption and urinary excretion of (-)-epicatechin after administration of different levels of cocoa powder or (-)-epicatechin in rats. J Agric Food Chem 2001; 49: 6050–6056.
Ramiro E, Franch A, Castellote C, Andres-Lacueva C, Izquierdo-Pulido M, Castell M . Effect of Theobroma cacao flavonoids on immune activation of a lymphoid cell line. Br J Nutr 2005; 93: 859–866.
Natsume M, Baba S . Suppressive effects of cacao polyphenols on the development of atherosclerosis in apolipoprotein E-deficient mice. Subcell Biochem 2014; 77: 189–198.
Villarreal-Calderon R, Reed W, Palacios-Moreno J, Keefe S, Herritt L, Brooks D, Torres-Jardon R, Calderon-Garciduenas L . Urban air pollution produces up-regulation of myocardial inflammatory genes and dark chocolate provides cardioprotection. Exp Toxicol Pathol 2012; 64: 297–306.
Qi GM, Jia LX, Li YL, Li HH, Du J . Adiponectin suppresses angiotensin II-induced inflammation and cardiac fibrosis through activation of macrophage autophagy. Endocrinology 2014; 155: 2254–2265; en20132011.
Tian C, Ye X, Zhang R, Long J, Ren W, Ding S, Liao D, Jin X, Wu H, Xu S, Ying C . Green tea polyphenols reduced fat deposits in high fat-fed rats via erk1/2-PPARgamma-adiponectin pathway. PLoS One 2013; 8: e53796.
Terra X, Montagut G, Bustos M, Llopiz N, Ardevol A, Blade C, Fernandez-Larrea J, Pujadas G, Salvado J, Arola L, Blay M . Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J Nutr Biochem 2009; 20: 210–218.
Ali F, Ismail A, Kersten S . Molecular mechanisms underlying the potential antiobesity-related diseases effect of cocoa polyphenols. Mol Nutr Food Res 2014; 58: 33–48.
Watanabe R, Azuma RW, Suzuki J, Ogawa M, Itai A, Hirata Y, Komuro I, Isobe M . Inhibition of NF-kappaB activation by a novel IKK inhibitor reduces the severity of experimental autoimmune myocarditis via suppression of T-cell activation. Am J Physiol Heart Circ Physiol 2013; 305: H1761–H1771.
Thandapilly SJ, Louis XL, Behbahani J, Movahed A, Yu L, Fandrich R, Zhang S, Kardami E, Anderson HD, Netticadan T . Reduced hemodynamic load aids low-dose resveratrol in reversing cardiovascular defects in hypertensive rats. Hypertens Res 2013; 36: 866–872.
Seko Y, Takahashi N, Azuma M, Yagita H, Okumura K, Yazaki Y . Effects of in vivo administration of anti-B7-1/B7-2 monoclonal antibodies on murine acute myocarditis caused by coxsackievirus B3. Circ Res 1998; 82: 613–618.
Sundaram A, Siew Keah L, Sirajudeen KN, Singh HJ . Upregulation of catalase and downregulation of glutathione peroxidase activity in the kidney precede the development of hypertension in pre-hypertensive SHR. Hypertens Res 2013; 36: 213–218.
Bing C, Bao Y, Jenkins J, Sanders P, Manieri M, Cinti S, Tisdale MJ, Trayhurn P . Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed in adipocytes and is up-regulated in mice with cancer cachexia. Proc Natl Acad Sci USA 2004; 101: 2500–2505.
Gensch N, Borchardt T, Schneider A, Riethmacher D, Braun T . Different autonomous myogenic cell populations revealed by ablation of Myf5-expressing cells during mouse embryogenesis. Development 2008; 135: 1597–1604.
Kaya Z, Afanasyeva M, Wang Y, Dohmen KM, Schlichting J, Tretter T, Fairweather D, Holers VM, Rose NR . Contribution of the innate immune system to autoimmune myocarditis: a role for complement. Nat Immunol 2001; 2: 739–745.
Odobasic D, Kitching AR, Semple TJ, Holdsworth SR . Endogenous myeloperoxidase promotes neutrophil-mediated renal injury, but attenuates T cell immunity inducing crescentic glomerulonephritis. J Am Soc Nephrol 2007; 18: 760–770.
Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J . Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 2010; 106: 1253–1264.
Nishioka S, Yoshioka T, Nomura A, Kato R, Miyamura M, Okada Y, Ishizaka N, Matsumura Y, Hayashi T . Celiprolol reduces oxidative stress and attenuates left ventricular remodeling induced by hypoxic stress in mice. Hypertens Res 2013; 36: 934–939.
Bedard K, Krause KH . The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 2007; 87: 245–313.
Afanasyeva M, Georgakopoulos D, Rose NR . Autoimmune myocarditis: cellular mediators of cardiac dysfunction. Autoimmun Rev 2004; 3: 476–486.
Smith SC, Allen PM . Myosin-induced acute myocarditis is a T cell-mediated disease. J Immunol 1991; 147: 2141–2147.
Maier R, Miller S, Kurrer M, Krebs P, de Giuli R, Kremer M, Scandella E, Ludewig B . Quantification and characterization of myosin peptide-specific CD4+ T cells in autoimmune myocarditis. J Immunol Methods 2005; 304: 117–125.
Al-Hanbali M, Ali D, Bustami M, Abdel-Malek S, Al-Hanbali R, Alhussainy T, Qadan F, Matalka KZ . Epicatechin suppresses IL-6, IL-8 and enhances IL-10 production with NF-kappaB nuclear translocation in whole blood stimulated system. Neuro Endocrinol Lett 2009; 30: 131–138.
Miyake M, Sasaki K, Ide K, Matsukura Y, Shijima K, Fujiwara D . Highly oligomeric procyanidins ameliorate experimental autoimmune encephalomyelitis via suppression of Th1 immunity. J Immunol 2006; 176: 5797–5804.
Baba S, Osakabe N, Natsume M, Terao J . Absorption and urinary excretion of procyanidin B2 [epicatechin-(4beta-8)-epicatechin] in rats. Free Radic Biol Med 2002; 33: 142–148.
Li Q, Verma IM . NF-kappaB regulation in the immune system. Nat Rev Immunol 2002; 2: 725–734.
Hiscott J, Marois J, Garoufalis J, D'Addario M, Roulston A, Kwan I, Pepin N, Lacoste J, Nguyen H, Bensi G, Fenton M . Characterization of a functional NF-kappa B site in the human interleukin 1 beta promoter: evidence for a positive autoregulatory loop. Mol Cell Biol 1993; 13: 6231–6240.
Blyszczuk P, Kania G, Dieterle T, Marty RR, Valaperti A, Berthonneche C, Pedrazzini T, Berger CT, Dirnhofer S, Matter CM, Penninger JM, Luscher TF, Eriksson U . Myeloid differentiation factor-88/interleukin-1 signaling controls cardiac fibrosis and heart failure progression in inflammatory dilated cardiomyopathy. Circ Res 2009; 105: 912–920.
Gloire G, Legrand-Poels S, Piette J . NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol 2006; 72: 1493–1505.
Leuschner F, Katus HA, Kaya Z . Autoimmune myocarditis: past, present and future. J Autoimmun 2009; 33: 282–289.
Tang Z, McGowan BS, Huber SA, McTiernan CF, Addya S, Surrey S, Kubota T, Fortina P, Higuchi Y, Diamond MA, Wyre DS, Feldman AM . Gene expression profiling during the transition to failure in TNF-alpha over-expressing mice demonstrates the development of autoimmune myocarditis. J Mol Cell Cardiol 2004; 36: 515–530.
Morgan MJ, Kim YS, Liu ZG . TNFalpha and reactive oxygen species in necrotic cell death. Cell Res 2008; 18: 343–349.
Acknowledgements
This study was supported by grants from Ryoushoku (the food science institute foundation), The Foundation for Dietary Scientific Research and the All Japan Coffee Association. We would like to thank Ms Noriko Tamura and Ms Yasuko Matsuda for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zempo, H., Suzuki, Ji., Watanabe, R. et al. Cacao polyphenols ameliorate autoimmune myocarditis in mice. Hypertens Res 39, 203–209 (2016). https://doi.org/10.1038/hr.2015.136
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2015.136
Keywords
This article is cited by
-
Gene expression changes by high-polyphenols cocoa powder intake: a randomized crossover clinical study
European Journal of Nutrition (2019)