Abstract
The mineralocorticoid receptor (MR) is expressed in the kidneys and in adipose tissue, and primary aldosteronism (PA) is associated with metabolic syndrome. This study assessed the effects of MR blockade by eplerenone (EPL) and spironolactone (SPL) on blood pressure (BP) and metabolic factors in patients with PA. Fifty-four patients with PA were treated with one of two MRAs, EPL (25–100 mg daily, n=27) or SPL (12.5–100 mg daily, n=27) for 12 months. Visceral (VAT) and subcutaneous adipose tissue were quantified using CT and FatScan imaging analysis software. Body mass index, homeostasis model assessment-insulin resistance (HOMA-IR), serum creatinine, potassium and lipids, urinary albumin excretion (UAE) and plasma aldosterone concentration (PAC) and plasma renin activity (PRA) were measured before and after treatment. EPL and SPL decreased BP and increased serum potassium levels to similar degrees. PAC and PRA did not differ between the two groups. Although treatment with the MRAs did not change HOMA-IR or serum lipids, they significantly decreased UAE and VAT (P<0.05). These results suggest that EPL and SPL are effective and safe for the treatment of PA. The long-term metabolic and renal effects of these MRAs should be further investigated.
Similar content being viewed by others
Introduction
Primary aldosteronism (PA) is the most common form of secondary hypertension, with an estimated prevalence of 5–15% in all hypertensive patients.1, 2 Excess aldosterone has adverse cardiovascular and renal consequences in patients with hypertension and in patients with diabetes mellitus or chronic renal disease.3, 4 We have previously reported that PA patients have a higher incidence of cardiovascular complications than age- and sex-matched patients with essential hypertension.5 Several studies have shown that PA is common in patients with resistant hypertension, with a prevalence of ~20%,6 and PA is associated with a significant increase in risk of end-organ damage and cardiovascular events compared with more easily controlled hypertension.7
Patients with aldosterone-producing adenoma (APA) or unilateral adrenal hyperplasia are often treated with unilateral laparoscopic adrenalectomy. Although surgical removal of APA improves hypertension by up to 30–50%,8 there are no data to show that adrenalectomy is superior to medical treatment. Spironolactone (SPL) and eplerenone (EPL), which both directly antagonize the mineralocorticoid receptor (MR), are the most appropriate therapeutic agents in patients with PA.9 SPL has been widely used for the treatment of PA, but its use is limited by adverse effects such as gynecomastia, mastodynia, menstrual abnormalities and impotence due to androgen receptor antagonist activity. EPL is a more specific MR antagonist (MRA) and has very few side effects.4
Patients with PA have a significantly higher prevalence of metabolic syndrome than those with essential hypertension.10, 11, 12 The MR is expressed in the kidneys and in adipose tissue. EPL improves obesity-related insulin resistance through the reduction of fat reactive oxygen species production, inflammatory processes and induction of cytokines in ob/ob mice.13 We examined the effects of MR blockade by EPL and SPL on blood pressure (BP), renal function and serum potassium and metabolic factors in patients with PA.
Methods
Subjects
The protocol and informed consent form were reviewed by the appropriate Independent Ethics Committee or Institutional Review Board before enrollment of any study patients. The clinical trial registration no. was UMIN000004581. To be included in the trial, men and non-childbearing women were at least 18 years of age with hypertension (seated diastolic BP⩾90 and ⩽120 mm Hg with systolic BP<200 mm Hg), serum potassium >3.0 and <5.0 mmol l−1. Exclusion criteria included history of accelerated/malignant hypertension, sex hormone therapy, reduced renal function (serum creatinine >1.5 mg dl−1 in men and 1.3 mg dl−1 in women), liver disease based on transaminases >2 times the upper limit of normal, and a history of heart failure, myocardial infarction, stroke or serious cardiovascular event within 6 months. Fifty-four hypertensive patients (24 men and 30 women; aged 56±10 years) were diagnosed with PA according to The Japanese Society of Endocrinology Guidelines for the Management of PA14 in the Kanazawa University Hospital and two municipal hospitals. Thirty-nine patients underwent adrenal venous sampling; nine subjects had unilateral aldosterone hypersecretion (four patients in the SPL group and five patients in the EPL group), and 30 subjects had bilateral production of aldosterone (17 patients in the SPL group and 13 patients in the EPL group). Adrenal venous sampling was not performed in 15 patients because they did not want to undergo the operation (six patients in the SPL group and nine patients in the EPL group).
Study design
This was an open-label, non-controlled study. After a 2-week washout period, patients with PA underwent randomization with the envelop method: 27 patients received SPL (25 mg) and 27 received EPL (50 mg) as the first dose. If BP was not at the goal level (<140/90 mm Hg) after 4 weeks of treatment, the dose of SPL or EPL was increased up to 100 mg. If BP remained uncontrolled after 8 weeks, a calcium channel blocker was added. The duration of hypertension was 7.7±8.9 (EPL group) and 6.0±8.7 years (SPL group). We assessed the visceral and subcutaneous fat area, insulin resistance as represented by homeostasis model assessment-insulin resistance, lipid parameters and urine albumin excretion at baseline and after MR blockade treatment. The study was performed in accordance with the principles of the Declaration of Helsinki, and the investigational protocol was approved by the Ethics Committee for Human Studies at the Kanazawa University Hospital. All patients provided written informed consent.
Laboratory measurements
Body mass index (BMI) was calculated as weight (kg) divided by height (cm) squared. Venous blood samples were obtained after a 12-h overnight fast after a 15 min rest in the supine position in the morning. Serum lipids, potassium, glucose and creatinine were determined by standard procedures. HbA1c was measured by high-pressure liquid chromatography. Urinary albumin was measured using a commercial enzyme-linked immunosorbent assay kit. Plasma immunoreactive insulin was measured using enzyme-linked immunosorbent assay, and blood glucose was measured using the glucose oxidase method. The insulin resistance index was calculated based on homeostasis model assessment-insulin resistance (fasting glucose (mg dl−1) × fasting insulin (mU ml−1)/405). The plasma aldosterone concentration, plasma renin activity and serum cortisol concentration were measured by radioimmunoassay as previously reported.15
Measurement of abdominal adipose tissue by CT
CT scans were performed using a 64-channel multi-detector row CT (MDCT; GE Healthcare, Little Chalfont, Buckinghamshire, England). All subjects underwent CT at the umbilical level to measure the cross-sectional abdominal subcutaneous adipose tissue area and visceral adipose tissue (VAT) area using FatScan software (N2 System Corp, Osaka, Japan).16 FatScan software enables multiple image rendering and geometric measurements of a specific region with a specified CT number (in Hounsfield units). A single cross-sectional scan at the level of the umbilicus was selected for quantification. Adipose tissue was determined by setting the attenuation level within the range of −190 to −30 Hounsfield units, and the acquired image corresponded to the total fat region. The region of visceral fat was defined by manual tracing of its contour, and the total fat region was divided into visceral and subcutaneous fat regions. Finally, the VAT was calculated by the software.
Statistics
Data are expressed as the means±s.d.’s. All of the analyses were conducted using SPSS software version 18.0 for Windows (SPSS, Chicago, IL, USA). Two-sided tests of treatment differences, based on analysis of covariance (ANOCOVA) with baseline as a covariate and treatment and center as factors were used to compare the effects of SPL and EPL.
Results
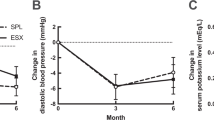
No significant differences in the clinical data were observed between the two groups (Table 1). There were 11 metabolic syndrome patients, three diabetic patients and 15 dyslipidemic patients in the SPL group at baseline. There were eight metabolic syndrome patients, four diabetic patients, 14 dyslipidemic patients in the EPL group at baseline. Additional antihypertensive drugs and other medications used at the end point are shown in Table 2. Two patients had successfully decreased BP with 25 mg per day of SPL, so their dose of SPL was reduced to 12.5 mg per day; these patients maintained their target BP. Table 3 summarizes the effects of MRAs on BP, plasma aldosterone concentration, plasma renin activity, serum potassium, renal function, and serum metabolic factors. Figure 1 shows treatment with SPL or EPL significantly decreased systolic BP and diastolic BP (P<0.001). Serum potassium levels did not exceed 5.0 m Eq l−1 in any patients. Estimeted glomerular filtration rate (eGFR) tended to decrease, but urinary albumin excretion (UAE) was significantly improved by MRA treatment (P=0.024). The differences between the effects of EPL and SPL on systolic and diastolic BP, plasma renin activity, plasma aldosterone concentration, serum potassium, eGFR, UAE and metabolic factors are shown in Table 3. The BP-lowering effects between the two agents did not differ. SPL significantly increased plasma aldosterone concentration compared with EPL (P=0.007). Plasma renin activity, serum potassium, eGFR and UAE did not differ between the two groups. The metabolic factors did not significantly differ between the two groups. Body weight, BMI, waist circumference, VAT and subcutaneous adipose tissue area were not different between the two groups. Although BMI and VAT area were significantly decreased in all patients (P<0.05), no significant differences in BMI, VAT and subcutaneous adipose tissue area were observed between the two groups. Two patients treated with SPL experienced gynecomastia. No patients treated with EPL showed gynecomastia.
Discussion
SPL has been successfully used in the treatment with PA for more than four decades as monotherapy or in combination with other antihypertensive drugs.9 EPL is approved for the treatment of essential hypertension in the United States and Japan, but not in Europe.17 Both EPL and SPL inhibit activation of the human MR, and SPL is more potent than EPL in blocking the aldosterone activation of MRs.18 Weinberger et al.19 reported that the BP reduction with SPL 50 mg b.i.d. was greater than with EPL 50 mg b.i.d. or 100 mg per day. There are limited data on the effects of EPL in patients with PA. Two recent studies compared the efficacy of EPL and SPL on BP reduction in patients with PA. Karagiannis et al.20 showed that treatment with EPL (from 50 to 200 mg per day) or SPL (from 50 to 400 mg per day) for 16 weeks in 34 patients with idiopathic hyperaldosteronism (IHA) resulted in an equally effective reduction in BP. Parthasarathy et al.21 reported that the antihypertensive effects of SPL (from 75 to 225 mg) were significantly greater than those of EPL (from 100 to 300 mg) in 141 patients with PA including APAs. Two studies reported the adverse events associated with SPL treatment, which included gynecomastia, breast pain in females and impotence. The early trials of SPL demonstrated its efficacy at doses up to over 200 mg, whereas lower doses of SPL have recently been used to avoid dose- and time-dependent adverse events while maintaining the drug’s antihypertensive effects.22, 23 Our data with lower doses of SPL or EPL might be more practical. Calcium channel blockers decrease aldosterone synthesis in human adrenocortical cells24 and in hypertensive patients.25 Nakamura et al.26 reported that EPL potentiates the protective effects of amlodipine against cardiovascular injury in salt-sensitive hypertensive rats independently of BP. In this study, 30–40% of patients needed to use amlodipine to achieve BP<140/90 mm Hg. Other BP-lowering agents such as ACE inhibitors, angiotensin II receptor blockers and thiazide diuretics were not used. Our data suggest that calcium channel blockers are useful add-ons for the treatment of PA.
(Table 3) Recent in vitro studies have suggested that aldosterone and the MR might influence adipocyte behavior. Caprio et al.27 reported that aldosterone promotes maturation of pre-adipocytes to adipocytes in a time-, dose- and MR-dependent manner. Briones et al.28 reported that adipocytes produce aldosterone, which regulates adipocyte differentiation and vascular function in an autocrine and paracrine manner. In addition, a higher prevalence of glycemic abnormalities and of the metabolic syndrome has been demonstrated in patients with PA compared with those with essential hypertension.29 Although a cause-effect relationship has not been established, the available evidence indicates that VAT might be a common element linking the many facets of the metabolic syndrome, including glucose intolerance, hypertension, dyslipidemia and insulin resistance.30 We found that treatment with MRAs decreased VAT but not subcutaneous adipose tissue in patients with PA; however, serum lipids and homeostasis model assessment-insulin resistance were not influenced by the medical treatment. Catena et al.11 reported that treatment with surgery or MRAs rapidly and persistently restores normal sensitivity to insulin in patients with PA, which was independent of plasma potassium levels. In their report, homeostasis model assessment-insulin resistance was decreased at 6 months after treatment and increased to the baseline levels over an average period of 5.7 years. Kosmala et al.31 reported that treatment with SPL in patients with metabolic syndrome resulted in a significant decrease in biomarkers of collagen synthesis, left atrial dimension, left ventricular wall thickness and mass. The beneficial effects of MRAs on cardiovascular and metabolic factors should be further investigated.
In this study, MR blockade therapy decreased UAE in patients with PA. We have previously reported that SPL reduced UAE in diabetic patients.32 Nagase et al.33 reported that EPL improved podocyte damage and retarded the progression of proteinuria and glomerulosclerosis. Iwakura et al.34 showed that in 111 patients with bilateral hyperaldosteronism treated with MRAs, UAE and eGFR were significantly decreased at 12 months after treatment; these results were consistent with the results from our study. Iwakura et al. noted that patients with higher UAE before treatment had the greatest reduction in eGFR. Recently, Ando et al.35 reported that the addition of low-dose EPL to renin-angiotensin system inhibitors had renoprotective effects through the reduction of albuminuria in non-diabetic hypertensive patients. A limitation of this current study is that in keeping with ethical practices, the study could not be controlled with a placebo. We did not provide enrolled subjects a special diet program to change their lifestyles, but the patients in both the SPL group and EPL group might have changed their lifestyles of their own volition. BMI decreases during treatment occurred, and lifestyles might have changed. Francis et al. showed that aldosterone stimulates salt appetite, and MRA administered intracerebroventricularly or intraperitoneally significantly reduced salt ingestion in congestive heart failure model rats.36
EPL was as effective as SPL for the treatment of hypertension in patients with PA and showed fewer side effects. The beneficial effect of both drugs on VAT is of interest and should be further investigated.
References
Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, Ganzaroli C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, Mannelli M, Mattarello MJ, Moretti A, Palumbo G, Parenti G, Porteri E, Semplicini A, Rizzoni D, Rossi E, Boscaro M, Pessina AC, Mantero F . PAPY Study Investigators. A prospective study of the prevalence of primary aldosteronsim in 1125 hypertensive patients. J Am Coll Cardiol 2006; 48: 2293–2300.
Ito Y, Takeda R, Karashima S, Yamamoto Y, Yoneda T, Takeda Y . Prevalence of primaryaldosteronism among prehypertensive and stage 1 hypertensive subjects. Hypertens Res 2011; 34: 98–102.
Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ . Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol 2005; 45: 1243–1245.
Takeda Y . Pleiotropic actions of aldosterone and the effects of eplerenone, a selective mineralocorticoid receptor antagonist. Hypertens Res 2004; 27: 781–789.
Takeda R, Matsubara T, Miyamori I, Hatakeyama H, Morise T . Vascular complications in patients with aldosterone producing adenoma in Japan: comparative study with essential hypertension. J Endocrinol Invest 1995; 18: 370–373.
Muiesan ML, Salvetti M, Rizzoni D, Paini A, Agabiti-Rosei C, Aggiusti C, Agabiti Rosei E . Resistant hypertension and target organ damage. Hypertens Res 2013; 36: 485–491.
Young WF . Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol 2007; 66: 607–618.
Quinkler M, Stewart PM . Treatment of primary aldosteronism. Best Pract Res Clin Endocrinol Metab 2010; 24: 923–932.
Colussi G, Catena C, Sechi LA . Spironolactone, eplerenone and the new aldosterone blockers in endocrine and primary aldosteronism. J Hypertens 2013; 31: 3–15.
Fallo F, Veglio F, Bertello C, Sonino N, Della Mea P, Ermani M, Rabbia F, Federspil G, Mulatero P . Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab 2006; 91: 454–459.
Catena C, Lapenna R, Baroselli S, Nadalini E, Colussi G, Novello M, Favret G, Melis A, Cavarape A, Sechi LA . Insulin sensitivity in patients with primary aldosteronism: a follow-up study. J Clin Endocrinol Metab 2006; 91: 3457–3463.
Giacchetti G, Ronconi V, Turchi F, Agostinelli L, Mantero F, Rilli S, Boscaro M . Aldosterone as a key mediator of the cardiometabolic syndrome in primary aldosteronism: an observational study. J Hypertens 2007; 25: 177–186.
Hirata A, Maeda N, Hiuge A, Hibuse T, Fujita K, Okada T, Kihara S, Funahashi T, Shimomura I . Blockade of mineralocorticoid receptor reverses adipocyte dysfunction and insulin resistance 1 in obese mice. Cardiovasc Res 2009; 84: 164–172.
Nishikawa T, Omura M, Satoh F, Shibata H, Takahashi K, Tamura N, Tanabe A . Task Force Committee on Primary Aldosteronism, The Japan Endocrine Society. Guidelines for the diagnosis and treatment of primary aldosteronism–the Japan Endocrine Society 2009. Endocr J 2011; 58: 711–721.
Takeda Y, Furukawa K, Inaba S, Miyamori I, Mabuchi H . Genetic analysis of aldosterone synthase in patients with idiopathic hyperaldosteronism. J Clin Endocrinol Metab 1999; 84: 1633–1637.
Yoshizumi T, Nakamura T, Yamane M, Islam AHMW, Menju M, Yamasaki K . Abdominal fat: standardized technique for measurement at CT. Radiology 1999; 211: 283–286.
Sato A . Mineralocorticoid receptor antagonists: their use and differentiation in Japan. Hypertens Res 2013; 36: 185–190.
Garthwaite SM, McMahon EG . The evolution of aldosterone antagonists. Mol Cell Endocrinol 2004; 217: 27–31.
Weinberger MH, Roniker B, Krause SL, Weiss RJ . Eplerenone, a selective aldosterone blocker, in mild-to-moderate hypertension. Am J Hypertens 2002; 15: 709–716.
Karagiannis A, Tziomalos K, Papageorgiou A, Kakafika AI, Pagourelias ED, Anagnostis P, Athyros VG, Mikhailidis DP . Spironolactone versus eplerenone for the treatment of idiopathic hyperaldosteronism. Expert Opin Pharmacother 2008; 9: 509–515.
Parthasarathy HK, Menard J, White WB, Young WF Jr, Williams GH, Williams B, Ruilope LM, McInnes GT, Connell JM, MacDonald TM . A double-blind, randomized study comparing the antihypertensive effect of eplerenone and spironolactone in patients with hypertension and evidence of primary aldosteronism. J Hypertens 2011; 29: 980–990.
Karagiannis A, Tziomalos K, Kakafika AI, Athyros VG, Harsoulis F, Mikhailidis DP . Medical treatment as an alternative to adrenalectomy in patients with aldosterone-producing adenomas. Endocr Relat Cancer 2008; 15: 693–700.
Jansen PM, Frenkel WJ, van den Born BJ, de Bruijne EL, Deinum J, Kerstens MN, Arnoldus JH, Woittiez AJ, Wijbenga JA, Zietse R, Danser AH, van den Meiracker AH . Determinants of blood pressure reduction by eplerenone in uncontrolled hypertension. J Hypertens 2013; 31: 404–413.
Isaka T, Ikeda K, Takada Y, Inada Y, Tojo K, Tajima N . Azelnidipine inhibits aldosterone synthesis and secretion in human adrenocortical cell line NCI-H295R. Eur J Pharmacol 2009; 605: 49–52.
Abe M, Okada K, Maruyama N, Matsumoto S, Maruyama T, Fujita T, Matsumoto K, Soma M . Benidipine reduces albuminuria and plasma aldosterone in mild-to-moderate stage chronic kidney disease with albuminuria. Hypertens Res 2011; 34: 268–273.
Nakamura T, Fukuda M, Kataoka K, Nako H, Tokutomi Y, Dong YF, Yamamoto E, Yasuda O, Ogawa H, Kim-Mitsuyama S . Eplerenone potentiates protective effects of amlodipine against cardiovascular injury in salt-sensitive hypertensive rats. Hypertens Res 2011; 34: 817–824.
Caprio M, Feve B, Claes A, Viengchareun S, Lombes M, Zennaro MC . Pivotal role of the mineralocorticoid receptor in corticosteroid-induced adipogenesis. FASEB J 2007; 21: 2185–2194.
Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, Correa JW, Gagnon AM, Gomez-Sanchez CE, Gomez-Sanchez EP, Sorisky A, Ooi TC, Ruzicka M, Burns KD, Touyz RM . Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension 2012; 59: 1069–1078.
Fallo F, Veglio F, Bertello C, Sonino N, Della Mea P, Ermani M, Rabbia F, Federspil G, Mulatero P . Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab 2006; 91: 454–459.
Sironi AM, Gastaldelli A, Mari A, Ciociaro D, Positano V, Buzzigoli E, Ghione S, Turchi S, Lombardi M, Ferrannini E . Visceral fat in hypertension, influence on insulin resistance and β-cell function. Hypertension 2004; 44: 127–133.
Kosmala W, Przewlocka-Kosmala M, Szczepanik-Osadnik H, Mysiak A, O'Moore-Sullivan T, Marwick TH . A randomized study of the beneficial effects of aldosterone antagonist on LV function, structure, and fibrosis markers in metabolic syndrome. JACC Cardiovasc Imaging 2011; 4: 1239–1249.
Yoneda T, Takeda Y, Usukura M, Oda N, Takata H, Yamamoto Y, Karashima S, Yamagishi M . Aldosterone breakthrough during angiotensin II receptor blockade in hypertensive patients with diabetes mellitus. Am J Hypertens 2007; 20: 1329–1333.
Nagase M, Shibata S, Yoshida S, Nagase T, Gotoda T, Fujita T . Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension 2006; 47: 1084–1093.
Iwakura Y, Morimoto R, Kudo M, Ono Y, Takase K, Seiji K, Arai Y, Nakamura Y, Sasano H, Ito S, Satoh F . Predictors of decreasing glomerular filtration rate and prevalence of chronic kidney disease after treatment of primary aldosteronism: renal outcome of 213 cases. J Clin Endocrinol Metab 2014; 99: 1593–1598.
Ando K, Ohtsu H, Uchida S, Kaname S, Arakawa Y, Fujita T . for the EVALUATE Study Group. Anti-albuminuric effect of the aldosterone blocker eplerenone in non-diabetic hypertensive patients with albuminuria: a double-blind, randomized, placebo-controlled trial. Lancet Diabetes Endocrinol 2014; 2: 944–953.
Francis J, Weiss RM, Wei SG, Johnson AK, Beltz TG, Zimmerman K, Felder RB . Central mineralocorticoid receptor blockade improves volume regulation and reduces sympathetic drive in heart failure. Am J Physiol Heart Circ Physiol 2001; 281: H2241–H2251.
Acknowledgements
This work was partially supported by the Health and Labor Sciences Research Grant of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
There have been no previous presentations of this work in its entirety or in part.
Rights and permissions
About this article
Cite this article
Karashima, S., Yoneda, T., Kometani, M. et al. Comparison of eplerenone and spironolactone for the treatment of primary aldosteronism. Hypertens Res 39, 133–137 (2016). https://doi.org/10.1038/hr.2015.129
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2015.129
Keywords
This article is cited by
-
A controlled trial of percutaneous adrenal arterial embolization for hypertension in patients with idiopathic hyperaldosteronism
Hypertension Research (2024)
-
Diagnosis and management of primary hyperaldosteronism in patients with hypertension: a practical approach endorsed by the British and Irish Hypertension Society
Journal of Human Hypertension (2023)
-
Recent progress in the diagnosis and treatment of primary aldosteronism
Hypertension Research (2023)
-
Long-term impact of spironolactone compliance on microalbuminuria in patients with primary aldosteronism
Hypertension Research (2021)
-
The mineralocorticoid receptor—an emerging player in metabolic syndrome?
Journal of Human Hypertension (2021)