Abstract
Because dementia is associated with both deterioration in the quality of life and poor prognosis, the prevention of cognitive impairment (CI) is a critical problem in public health promotion. Hypertension is a risk factor for the aggravation of CI, and the renin–angiotensin–aldosterone system (RAAS) is a key player in the increased incidence and development of hypertension. Therefore, the RAAS is considered to be a promoting factor for CI development. Conversely, recent studies have shown that lowering blood pressure with RAAS inhibitors decreases the incidence of CI, dementia and cardiovascular disease. Blood–brain barrier-penetrating RAAS inhibitors appear to have advantages in preventing cognitive decline because they can suppress the RAAS in the hippocampus, which has an important role in cognition. Thus, RAAS blockage is a notable strategy for preventing CI.
Similar content being viewed by others
Introduction
Due to the aging of the population, the number of elderly patients with dementia is increasing. Dementia is associated with both deterioration in the quality of life and poor prognosis; the prevention of dementia is therefore important for promoting public health and reducing national medical expenses.1 Dementia includes Alzheimer’s disease (AD) and vascular dementia; the major causes of these two types of dementia are hypothesized to be amyloid β (Aβ) deposition due to a disorder of cholinergic neurons and vascular injury, respectively. Recent studies have shown that the manifestation of dementia can be delayed by several pharmacological agents, including cholinesterase inhibitors;2 however, sufficient treatment for patients with dementia has not yet been established. Because it is difficult to remove deposits of Aβ at an advanced stage, the early detection of cognitive impairment (CI) by screening and identifying high-risk patients are important issues.
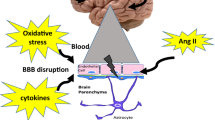
Hypertension is hypothesized to be one of the most critical risk factors for CI, and the renin–angiotensin–aldosterone system (RAAS) is a key player for hypertension in a blood pressure (BP)-dependent/independent manner. Therefore, the RAAS is a crucial target for preventing CI (Figure 1). In this review, we focus on effects of the RAAS on CI leading to dementia.
Hypertension is a risk factor for dementia and cognitive impairment
It is unclear whether there is an association between hypertension and cognitive decline; however, some longitudinal cohort studies have revealed there is a positive association between these conditions.3, 4, 5
Dementia can be classified into two types based on the pathogenesis: AD and vascular dementia. AD is a neurodegenerative disease caused by damage to cholinergic neurons, which leads to Aβ peptide deposition, especially in the cerebral limbic system, including the hippocampus. On the other hand, vascular dementia is caused by neural damage from a single stroke or multiple strokes, interrupting brain circulation for memory and cognition. Vascular dementia also results from damage to subcortical small vessels of the medullary arteries due to exposure to highly pulsatile pressure and flow, which causes white matter damage, lacunae and loss of cortical connections.
In some cases, these two types of dementia overlap. Accumulating evidence suggests associations among AD, cardiovascular risk factors and atherosclerosis. Neuroimaging and postmortem histopathological studies have indicated that up to one third of AD patients have some degree of vascular pathology; AD lesions are also present in a similar proportion of vascular dementia patients.6, 7 Moreover, decreased cerebral blood flow is a common and early observation in people with AD, implying that Aβ induces vascular dysfunction.8, 9
Hypertension is associated with increased risk for CI, leading to AD in addition to vascular dementia, suggesting that lowering BP would reduce the incidence of dementia. Petrovitch et al.10 showed that elevated systolic BP in midlife was associated with low brain weight and greater numbers of neurofibrillary tangles of hyperphosphorylated tau proteins, known as a primary marker of AD, in both the neocortex and hippocampus. In addition, diastolic BP elevation has been shown to be associated with greater numbers of neurofibrillary tangles in the hippocampus. Kivipelto et al.11, 12, 13 showed that high systolic BP is also a risk factor for AD in later life. Posner et al.14 showed that a history of hypertension is not associated with AD incidence, but is associated with an increased risk of vascular dementia, particularly in the presence of heart disease or diabetes. These studies indicate high BP is a risk for both vascular dementia and AD.
Moreover, high BP is associated with not only dementia but also with cognitive dysfunction, an early manifestation of dementia, especially in the elderly. Cross-sectional studies have shown that systolic and/or diastolic BP were associated with declined cognitive function.15, 16, 17, 18, 19, 20, 21 Longitudinal studies have shown that high BP was significantly associated with the decline in visualization/fluid abilities in both younger and older age groups.22 On the other hand, some studies have shown that a certain BP level, particularly a systolic pressure of at least 130 mm Hg, is important for the maintenance of cognitive functioning in the elderly.23, 24 Thus, lowering BP at an old age does not prevent dementia and lowering BP beginning in the midlife period therefore appears to be an important strategy for preserving cognitive function.
Lowering BP ameliorates dementia and cognitive impairment
Several studies have shown that lowering BP is associated with a decline of cognitive dysfunction or incidence of dementia,25 although other studies have failed to show a relationship between BP lowering and cognitive function.26, 27 The Syst-Eur study demonstrated that antihypertensive treatment with nitrendipine, a calcium channel blocker, in elderly patients reduced the incidence of dementia; the reduction in AD was greater than that of vascular dementia.28 The hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG) trial showed that antihypertensive treatment with indapamide, a diuretic, in elderly patients did not significantly reduce the incidence of dementia;29 however, significant favorable effects of antihypertensive treatment were observed when these data were combined in a meta-analysis with data from other placebo-controlled trials of antihypertensive treatment, and the combined risk ratio favored treatment.29 Therefore, lowering BP appears to have a role in preventing dementia and in preventing cardiovascular events. Conversely, dementia per se could be an independent cardiovascular risk factor: patients with dementia have lifestyle-related problems, such as inappropriate food or alcohol intake, sedentary activity levels and psychosocial stress, including depression.30 Therefore, preventing dementia might lead to the suppression of cardiovascular events.
RAAS is involved in cognitive decline
Several animal studies have shown that RAAS activation is associated with CI. RAAS-activated model mice showed declined cognitive function compared with wild-type mice, and the declined cognitive function was associated with a decrease in cerebral surface blood flow and an increase in oxidative stress.31, 32
Systemic RAAS activation occurs when angiotensinogen is cleaved in circulation by renin, which is secreted by the kidney, to form angiotensin (Ang) I. Ang I is then converted to Ang II by angiotensin-converting enzyme (ACE) in the pulmonary circulation. Ang II activates Ang II type 1 receptors on vascular smooth muscle cells to induce vasoconstriction. Ang II also stimulates the release of aldosterone from the adrenal cortex into circulation. In addition, the local RAAS is believed to have a pivotal role in organ damage. Components of the RAAS have been detected in various organs, such as the heart, kidney and brain. Indeed, the brain expresses genes that encode all components of the RAAS.33, 34, 35 The local RAAS can be activated independently of the systemic RAAS, indicating that end-organ damage may occur even though the systemic RAAS is attenuated.
Therefore, local RAAS blockage is necessary to prevent CI in addition to a systemic RAAS blockage; however, controversy remains regarding, which drugs are useful for blocking the brain RAAS. Recent studies have indicated that a drug’s ability to prevent CI depends on blood–brain barrier (BBB) penetration, and is independent of BP-lowering effects (Table 1). Of note, although there is no evidence of penetration through the BBB, some RAAS inhibitors have clinical benefits for preventing CI, suggesting that RAAS inhibitors might pass through the damaged BBB caused by the accumulation of cardiorenal risk factors.
In addition, diabetes is an independent risk factor for dementia and hypertension because the RAAS is also activated in diabetic patients. Blockage of the RAAS in diabetic patients might therefore be effective for preventing dementia, even in patients without hypertension.36
ACE inhibitors
The Syst-Eur study demonstrated that active treatment with an ACE inhibitor (ACE-I) (perindopril) with possible addition of a diuretic (indapamide) was associated with reduced risks of dementia and cognitive decline associated with recurrent stroke.37 It has been shown that brain-penetrating ACE-Is suppress cognitive decline compared with non-brain-penetrating ACE-Is or calcium channel blockers,38, 39 which is consistent with other observational studies.40 Therefore, blockage of the brain RAAS with brain-penetrating drugs is important to cognitive function and systemic blockage of the RAAS.
The PROGRESS (Perindopril Protection Against Recurrent Stroke study) study showed that an ACE-I (perindopril) with possible addition of a diuretic (indapamide) reduced the risks of dementia and cognitive decline associated with recurrent stroke.31 The HOPE (Heart Outcomes Prevention Evaluation) trial showed that an ACE-I (ramipril) reduced the incidence of stroke and the rate of CI in patients at high risk, despite only a modest reduction in BP.41
An increase in ACE activity has been shown in AD, indicating that ACE-Is are effective for preventing AD. ACE has been detected in the cerebral parenchyma, including microglia, astrocytes and vasculature of the brain, especially in the endothelium.42 BBB-penetrating ACE-Is can inhibit the activity of hippocampal ACE and prevent cognitive decline.43 There appear to be some possible mechanisms by which ACE-Is prevent CI. ACE-Is reduce oxidative stress, which can cause neuron damage.44 Inhibition of ACE activity in the brain also enhances the release of acetylcholine from neurons. Moreover, ACE inhibition decreases deposition of Aβ through an increase in substance P, which can activate neutral endopeptidase, an enzyme that degrades Aβ in the brain.45, 46 ACE-Is, especially BBB-penetrating ACE-Is, are able to prevent cognitive decline.
Angiotensin II receptor blockers
In addition, an angiotensin II receptor blocker (ARB) improved CI through a decrease in hippocampal long-term potentiation and improvement in functional blood flow compared with a calcium channel blocker.47
Some studies have shown that inhibition of the RAAS improves not only vascular function but also the accumulation of Aβ. Wang et al.48 reported that certain ARBs reduced Aβ accumulation and attenuated the development of Aβ-mediated cognitive deterioration through a possible efflux of Aβ from the brain into circulation. Hajjar et al.49 showed in an autopsy study that treatment with ARBs is associated with less Aβ accumulation and AD-related pathology independent of other AD risk factors.
The OSCAR (OlmeSartan and Calcium Antagonists Randomized) study demonstrated that significant systolic BP reduction with an ARB (eprosartan) had a negative association with cognitive decline.50 Fogari et al.51 reported that a group of patients treated with an ARB (telmisartan) and diuretic (hydrochlorothiazide) showed significantly improved cognitive function compared with a group treated with an ACE-I (lisinopril) and a diuretic (hydrochlorothiazide). Li et al.12 reported that treatments with ARBs were associated with a significant reduction in the incidence and progression of AD and dementia compared with treatments with ACE-Is or other cardiovascular drugs in a predominantly male population.
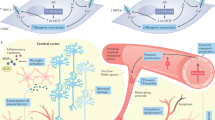
There appear to be some mechanisms involved in the ability of ARBs to prevent CI in addition to reducing oxidative stress. Mogi et al.52 showed that the angiotensin II type-2 (AT2) receptor has an important role in brain protection because cognitive function was significantly impaired in AT2 receptor-null mice compared with that in wild-type mice, and direct stimulation of the AT2 receptor by a newly generated direct AT2 receptor agonist, Compound 21, enhanced cognitive function in wild-type mice, which was not observed in AT2 receptor-deficient mice.53 Ang IV, a degradation product of Ang II, might have a role in CI prevention because the Ang IV receptor has an important role in memory in the hippocampus.44 AT receptors, including AT1, AT2 and AT4, have been detected in the hippocampus; therefore, blockage of AT1 and enhancement of AT2 and AT4 signaling by an ARB might be a reasonable strategy for preventing CI (Figure 2).54
Protective effects of renin–angiotensin–aldosterone system (RAAS) inhibitors on hippocampal neuron and vascular damage. Ang, angiotensin; Ald, aldosterone; MR, mineralocorticoid receptor; I, inhibitors; ARB, angiotensin II receptor blockers. A full color version of this figure is available at the Hypertension Research journal online.
Mineralocorticoid receptor blockers
It is well known that an increased plasma aldosterone level is a risk factor for the development of cardiovascular diseases.55 In addition, the incidence of cerebrovascular disease was shown to be significantly higher in patients with primary aldosteronism than in an essential hypertension group.56 A number of previous studies have shown that blockage of the mineralocorticoid receptor (MR) prevents cerebrovascular events, and that MR blockers improve cognitive function and prognosis in a BP-independent manner.57, 58, 59 The Cache County cohort study in Utah, USA, showed that potassium-sparing diuretics are associated with reduced incidence of AD compared with other antihypertensive drugs, suggesting that potassium-sparing diuretics, including spironolactone, have additive effects on dementia prevention.60
Iwanami et al.61 showed that MR blockage by eplerenone has a protective effect on ischemic brain damage through improved cerebral blood flow in the penumbra and reduction of oxidative stress in wild-type mice. MR blockers reduce the size of cerebral infarcts via a reduction in the expression of epidermal growth factor receptor mRNA, leading to reduced remodeling in stroke-prone, spontaneously hypertensive rats.59 We previously showed that a high plasma aldosterone level is a risk factor for CI, and that the administration of an MR blocker prevents further CI in patients with hypertension.62 Sakata et al.63 demonstrated that MR blockage with spironolactone improved impaired cognitive function observed in diabetic female mice in a BP-independent manner.
The MR has been identified not only in blood vessels but also in the brain, especially in the hippocampus. Moreover, aldosterone is synthesized in the brain and enters the brain from circulation. Therefore, blockage of aldosterone in the brain is critical to prevent CI in addition to systemic RAAS inhibition. Activation of the RAAS or low potassium concentration has been suggested to be involved in CI through possible contributions to CI pathogenesis, including oxidative stress, inflammation, platelet aggregation and vasoconstriction.64
Renin inhibitor
Aliskiren, a direct renin inhibitor, ameliorated brain damage and working memory deficits in a model of chronic cerebral ischemia through oxidative stress attenuation.65 Inhibition of upstream RAAS components could prevent the synthesis of all types of angiotensins, and might be a strategy to prevent cognitive decline; however, more evidence is needed to confirm aliskiren’s ability to prevent CI.
Conclusion
Activation of the RAAS is associated with cognitive decline and the development of hypertension. The RAAS in the brain might be an important target for the prevention of CI.
References
Hoshide S, Ishikawa J, Eguchi K, Oowada T, Shimada K, Kario K . Cognitive dysfunction and physical disability are associated with mortality in extremely elderly patients. Hypertens Res 2008; 31: 1331–1338.
Uzun S, Kozumplik O, Folnegovic-Smalc V . Alzheimer’s dementia: current data review. Coll Antropol 2011; 35: 1333–1337.
Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ . The association between midlife blood-pressure levels and late-life cognitive function—the Honolulu-Asia Aging Study. JAMA 1995; 274: 1846–1851.
Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, Persson G, Oden A, Svanborg A . 15-year longitudinal study of blood pressure and dementia. Lancet 1996; 347: 1141–1145.
Yoshitake T, Kiyohara Y, Kato I, Ohmura T, Iwamoto H, Nakayama K, Ohmori S, Nomiyama K, Kawano H, Ueda K, Sueishi K, Tsuneyoshi M, Fujishima M . Incidence and risk-factors of vascular dementia and Alzheimers-disease in a defined elderly Japanese population—the Hisayama Study. Neurology 1995; 45: 1161–1168.
Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA . Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology 2004; 62: 1148–1155.
Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR . Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA 1997; 277: 813–817.
Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MM . Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol 2005; 57: 789–794.
Prohovnik I, Mayeux R, Sackeim HA, Smith G, Stern Y, Alderson PO . Cerebral perfusion as a diagnostic marker of early Alzheimer’s disease. Neurology 1988; 38: 931–937.
Petrovitch H, White LR, Izmirilian G, Ross GW, Havlik RJ, Markesbery W, Nelson J, Davis DG, Hardman J, Foley DJ, Launer LJ . Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu-Asia aging Study. Neurobiol Aging 2000; 21: 57–62.
Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A . Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ 2001; 322: 1447–1451.
Li NC, Lee A, Whitmer RA, Kivipelto M, Lawler E, Kazis LE, Wolozin B . Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ 2010; 340: b5465.
Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, Nissinen A . Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 2005; 62: 1556–1560.
Posner HB, Tang MX, Luchsinger J, Lantigua R, Stern Y, Mayeux R . The relationship of hypertension in the elderly to AD, vascular dementia, and cognitive function. Neurology 2002; 58: 1175–1181.
Starr JM, Whalley LJ, Inch S, Shering PA . Blood pressure and cognitive function in healthy old people. J Am Geriatr Soc 1993; 41: 753–756.
Kuusisto J, Koivisto K, Mykkanen L, Helkala EL, Vanhanen M, Hanninen T, Pyorala K, Riekkinen P, Laakso M . Essential hypertension and cognitive function. The role of hyperinsulinemia. Hypertension 1993; 22: 771–779.
Suhr JA, Stewart JC, France CR . The relationship between blood pressure and cognitive performance in the third national health and nutrition examination survey (NHANES III). Psychosom Med 2004; 66: 291–297.
Robbins MA, Elias MF, Elias PK, Budge MM . Blood pressure and cognitive function in an African-American and a Caucasian-American sample: the Maine-Syracuse Study. Psychosom Med 2005; 67: 707–714.
Obisesan TO, Obisesan OA, Martins S, Alamgir L, Bond V, Maxwell C, Gillum RF . High blood pressure, hypertension, and high pulse pressure are associated with poorer cognitive function in persons aged 60 and older: the Third National Health and Nutrition Examination Survey. J Am Geriatr Soc 2008; 56: 501–509.
Cacciatore F, Abete P, Ferrara N, Paolisso G, Amato L, Canonico S, Maggi S, Rengo F . The role of blood pressure in cognitive impairment in an elderly population. J Hypertens 1997; 15: 135–142.
Kilander L, Nyman H, Boberg M, Hansson L, Lithell H . Hypertension is related to cognitive impairment—A 20-year follow-up of 999 men. Hypertension 1998; 31: 780–786.
Elias PK, Elias MF, Robbins MA, Budge MM . Blood pressure-related cognitive decline—Does age make a difference? Hypertension 2004; 44: 631–636.
Guo ZC, Fratiglioni L, Winblad B, Viitanen M . Blood pressure and performance on the Mini-Mental State Examination in the very old—Cross-sectional and longitudinal data from the Kungsholmen Project. Am J Epidemiol 1997; 145: 1106–1113.
Waldstein SR, Giggey PP, Thayer JF, Zonderman AB . Nonlinear relations of blood pressure to cognitive function—The Baltimore Longitudinal Study of Aging. Hypertension 2005; 45: 374–379.
Nagai M, Hoshide S, Kario K . Hypertension and Dementia. Am J Hypertens 2010; 23: 116–124.
Prince MJ, Bird AS, Blizard RA, Mann AH . Is the cognitive function of older patients affected by antihypertensive treatment? Results from 54 months of the Medical Research Council's treatment trial of hypertension in older adults. BMJ 1996; 312: 801–805.
Applegate WB, Pressel S, Wittes J, Luhr J, Shekelle RB, Camel GH, Greenlick MR, Hadley E, Moye L, Perry HM Jr et al. Impact of the treatment of isolated systolic hypertension on behavioral variables. Results from the systolic hypertension in the elderly program. Arch Intern Med 1994; 154: 2154–2160.
Forette F, Seux ML, Staessen JA, Thijs L, Birkenhager WH, Babarskiene MR, Babeanu S, Bossini A, Gil-Extremera B, Girerd X, Laks T, Lilov E, Moisseyev V, Tuomilehto J, Vanhanen H, Webster J, Yodfat Y, Fagard R . Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet 1998; 352: 1347–1351.
Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, Waldman A, Walton I, Poulter R, Ma S, Comsa M, Burch L, Fletcher A, Bulpitt C . Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol 2008; 7: 683–689.
O’Donnell M, Teo K, Gao P, Anderson C, Sleight P, Dans A, Marzona I, Bosch J, Probstfield J, Yusuf S . Cognitive impairment and risk of cardiovascular events and mortality. Eur Heart J 2012; 33: 1777–1786.
Tzourio C, Anderson C, Chapman N, Woodward M, Neal B, MacMahon S, Chalmers J . Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med 2003; 163: 1069–1075.
Inaba S, Iwai M, Furuno M, Tomono Y, Kanno H, Senba I, Okayama H, Mogi M, Higaki J, Horiuchi M . Continuous activation of renin-angiotensin system impairs cognitive function in human renin/angiotensinogen transgenic mice. Hypertension 2008; 52: E108–E109.
Lenkei Z, Corvol P, Llorenscortes C . The angiotensin receptor subtype At1a predominates in rat forebrain areas involved in blood-pressure, body-fluid homeostasis and neuroendocrine control. Brain Res Mol Brain Res 1995; 30: 53–60.
Phillips MI, Sumners C . Angiotensin II in central nervous system physiology. Regul Pep 1998; 78: 1–11.
Veerasingham SJ, Raizada MK . Brain renin-angiotensin system dysfunction in hypertension: recent advances and perspectives. Br J Pharmacol 2003; 139: 191–202.
Johnson ML, Parikh N, Kunik ME, Schulz PE, Patel JG, Chen H, Aparasu RR, Morgan RO . Antihypertensive drug use and the risk of dementia in patients with diabetes mellitus. Alzheimers Dement 2012; 8: 437–444.
Forette F, Seux ML, Staessen JA, Thijs L, Babarskiene MR, Babeanu S, Bossini A, Fagard R, Gil-Extremera B, Laks T, Kobalava Z, Sarti C, Tuomilehto J, Vanhanen H, Webster J, Yodfat Y, Birkenhager WH . The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Arch Intern Med 2002; 162: 2046–2052.
Ohrui T, Tomita N, Sato-Nakagawa T, Matsui T, Maruyama M, Niwa K, Arai H, Sasaki H . Effects of brain-penetrating ACE inhibitors on Alzheimer disease progression. Neurology 2004; 63: 1324–1325.
Ohrui T, Matsui T, Yamaya M, Arai H, Ebihara S, Maruyama M, Sasaki H . Angiotensin-converting enzyme inhibitors and incidence of Alzheimer’s disease in Japan. J Am Geriatr Soc 2004; 52: 649–650.
Rozzini L, Chilovi BV, Bertoletti E, Conti M, Del Rio I, Trabucchi M, Padovani A . Angiotensin converting enzyme (ACE) inhibitors modulate the rate of progression of amnestic mild cognitive impairment. Int J Geriatr Psychiatry 2006; 21: 550–555.
Bosch J, Yusuf S, Pogue J, Sleight P, Lonn E, Rangoonwala B, Davies R, Ostergren J, Probstfield J, Investigators H . Use of ramipril in preventing stroke: double blind randomised trial. BMJ 2002; 324: 699–702.
Pihlaja R, Koistinaho J, Kauppinen R, Sandholm J, Tanila H, Koistinaho M . Multiple cellular and molecular mechanisms are involved in human Abeta clearance by transplanted adult astrocytes. Glia 2011; 59: 1643–1657.
Dong YF, Kataoka K, Tokutomi Y, Nako H, Nakamura T, Toyama K, Sueta D, Koibuchi N, Yamamoto E, Ogawa H, Kim-Mitsuyama S . Perindopril, a centrally active angiotensin-converting enzyme inhibitor, prevents cognitive impairment in mouse models of Alzheimer’s disease. FASEB J 2011; 25: 2911–2920.
Ciobica A, Bild W, Hritcu L, Haulica I . Brain renin-angiotensin system in cognitive function: pre-clinical findings and implications for prevention and treatment of dementia. Acta Neurologica Belgica 2009; 109: 171–180.
Kehoe PG, Wilcock GK . Is inhibition of the renin-angiotensin system a new treatment option for Alzheimer’s disease? Lancet Neurology 2007; 6: 373–378.
Phillips MI, de Oliveira EM . Brain renin angiotensin in disease. J Mol Med (Berl) 2008; 86: 715–722.
Takeda S, Sato N, Takeuchi D, Kurinami H, Shinohara M, Niisato K, Kano M, Ogihara T, Rakugi H, Morishita R . Angiotensin receptor blocker prevented beta-amyloid-induced cognitive impairment associated with recovery of neurovascular coupling. Hypertension 2009; 54: 1345–U1125.
Wang J, Ho L, Chen LH, Zhao Z, Zhao W, Qian XJ, Humala N, Seror I, Bartholomew S, Rosendorff C, Pasinetti GM . Valsartan lowers brain beta-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J Clin Invest 2007; 117: 3393–3402.
Hajjar I, Brown L, Mack WJ, Chui H . Impact of angiotensin receptor blockers on Alzheimer disease neuropathology in a large brain autopsy series. Arch Neurol 2012, 1–7.
Hanon O, Berrou JP, Negre-Pages L, Goch JH, Nadhazi Z, Petrella R, Sedefdjian A, Sevenier F, Shlyakhto EV, Pathak A . Effects of hypertension therapy based on eprosartan on systolic arterial blood pressure and cognitive function: primary results of the Observational Study on Cognitive function And Systolic Blood Pressure Reduction open-label study. J Hypertens 2008; 26: 1642–1650.
Fogari R, Mugellini A, Zoppi A, Lazzari P, Destro M, Rinaldi A, Preti P . Effect of telmisartan/hydrochlorothiazide vs lisinopril/hydrochlorothiazide combination on ambulatory blood pressure and cognitive function in elderly hypertensive patients. J Hum Hypertens 2006; 20: 177–185.
Mogi M, Li JM, Iwanami J, Min LJ, Tsukuda K, Iwai M, Horiuchi M . Angiotensin II type-2 receptor stimulation prevents neural damage by transcriptional activation of methyl methanesulfonate sensitive 2. Hypertension 2006; 48: 141–148.
Jing F, Mogi M, Sakata A, Iwanami J, Tsukuda K, Ohshima K, Min LJ, Steckelings UM, Unger T, Dahlof B, Horiuchi M . Direct stimulation of angiotensin II type 2 receptor enhances spatial memory. J Cereb Blood Flow Metab 2012; 32: 248–255.
von Bohlen und Halbach O, Albrecht D . The CNS renin-angiotensin system. Cell Tissue Res 2006; 326: 599–616.
Conn JW, Knopf RF, Nesbit RM . Clinical characteristics of primary aldosteronism from an analysis of 145 cases. Am J Surg 1964; 107: 159–172.
Takeda R, Matsubara T, Miyamori I, Hatakeyama H, Morise T . Vascular complications in patients with aldosterone producing adenoma in Japan: comparative study with essential hypertension. The Research Committee of Disorders of Adrenal Hormones in Japan. J Endocrinol Invest 1995; 18: 370–373.
MacLeod AB, Vasdev S, Smeda JS . The role of blood pressure and aldosterone in the production of hemorrhagic stroke in captopril-treated hypertensive rats. Stroke 1997; 28: 1821–1828 discussion 1829.
Rocha R, Stier CT Jr . Pathophysiological effects of aldosterone in cardiovascular tissues. Trends Endocrinol Metab 2001; 12: 308–314.
Dorrance AM, Osborn HL, Grekin R, Webb RC . Spironolactone reduces cerebral infarct size and EGF-receptor mRNA in stroke-prone rats. Am J Physiol Regul Integr Comp Physiol 2001; 281: R944–R950.
Khachaturian AS, Zandi PP, Lyketsos CG, Hayden KM, Skoog I, Norton MC, Tschanz JT, Mayer LS, Welsh-Bohmer KA, Breitner JC . Antihypertensive medication use and incident Alzheimer disease: the Cache County Study. Arch Neurol 2006; 63: 686–692.
Iwanami J, Mogi M, Okamoto S, Gao XY, Li JM, Min LJ, Ide A, Tsukuda K, Iwai M, Horiuchi M . Pretreatment with eplerenone reduces stroke volume in mouse middle cerebral artery occlusion model. Eur J Pharmacol 2007; 566: 153–159.
Yagi S, Akaike M, Aihara K, Iwase T, Yoshida S, Sumitomo-Ueda Y, Ikeda Y, Ishikawa K, Matsumoto T, Sata M . High plasma aldosterone concentration is a novel risk factor of cognitive impairment in patients with hypertension. Hypertens Res 2011; 34: 74–78.
Sakata A, Mogi M, Iwanami J, Tsukuda K, Min LJ, Jing F, Ohshima K, Ito M, Horiuchi M . Improvement of cognitive impairment in female type 2 diabetes mellitus mice by spironolactone. J Renin Angiotensin Aldosterone Syst 2012; 13: 84–90.
Ito S, Komatsu K, Yajima Y, Hirayama A . Resin-angiotensin system in the brain as a new target of antihypertensive therapy. Hypertens Res 2008; 31: 1487–1488.
Dong YF, Kataoka K, Toyama K, Sueta D, Koibuchi N, Yamamoto E, Yata K, Tomimoto H, Ogawa H, Kim-Mitsuyama S . Attenuation of brain damage and cognitive impairment by direct renin inhibition in mice with chronic cerebral hypoperfusion. Hypertension 2011; 58: 635–642.
Unger T . Inhibiting angiotensin receptors in the brain: possible therapeutic implications. Curr Med Res Opin 2003; 19: 449–451.
Kim-Mitsuyama S, Yamamoto E, Tanaka T, Zhan Y, Izumi Y, Izumiya Y, Ioroi T, Wanibuchi H, Iwao H . Critical role of angiotensin II in excess salt-induced brain oxidative stress of stroke-prone spontaneously hypertensive rats. Stroke 2005; 36: 1083–1088.
Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, Trenkwalder P, Zanchetti A . The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003; 21: 875–886.
Skoog I, Lithell H, Hansson L, Elmfeldt D, Hofman A, Olofsson B, Trenkwalder P, Zanchetti A . Effect of baseline cognitive function and antihypertensive treatment on cognitive and cardiovascular outcomes: Study on COgnition and Prognosis in the Elderly (SCOPE). Am J Hypertens 2005; 18: 1052–1059.
Saxby BK, Harrington F, Wesnes KA, McKeith IG, Ford GA . Candesartan and cognitive decline in older patients with hypertension: a substudy of the SCOPE trial. Neurology 2008; 70 (19 Pt 2): 1858–1866.
Fogari R, Mugellini A, Zoppi A, Marasi G, Pasotti C, Poletti L, Rinaldi A, Preti P . Effects of valsartan compared with enalapril on blood pressure and cognitive function in elderly patients with essential hypertension. Eur J Clin Pharmacol 2004; 59: 863–868.
Gohlke P, Weiss S, Jansen A, Wienen W, Stangier J, Rascher W, Culman J, Unger T . AT1 receptor antagonist telmisartan administered peripherally inhibits central responses to angiotensin II in conscious rats. J Pharmacol Exp Ther 2001; 298: 62–70.
Noda A, Fushiki H, Murakami Y, Sasaki H, Miyoshi S, Kakuta H, Nishimura S . Brain penetration of telmisartan, a unique centrally acting angiotensin II type 1 receptor blocker, studied by PET in conscious rhesus macaques. Nucl Med Biol 2012; 39: 1232–1235.
Kishi T, Hirooka Y, Sunagawa K . Telmisartan protects against cognitive decline via up-regulation of brain-derived neurotrophic factor/tropomyosin-related kinase B in hippocampus of hypertensive rats. J Cardiol 2012; 60: 489–494.
Min LJ, Mogi M, Shudou M, Jing F, Tsukuda K, Ohshima K, Iwanami J, Horiuchi M . Peroxisome proliferator-activated receptor-gamma activation with angiotensin II type 1 receptor blockade is pivotal for the prevention of blood-brain barrier impairment and cognitive decline in type 2 diabetic mice. Hypertension 2012; 59: 1079–1088.
Kume K, Hanyu H, Sakurai H, Takada Y, Onuma T, Iwamoto T . Effects of telmisartan on cognition and regional cerebral blood flow in hypertensive patients with Alzheimer’s disease. Geriatr Gerontol Int 2012; 12: 207–214.
Matsumoto S, Shimodozono M, Miyata R, Kawahira K . Effect of the angiotensin II type 1 receptor antagonist olmesartan on cerebral hemodynamics and rehabilitation outcomes in hypertensive post-stroke patients. Brain Inj 2009; 23: 1065–1072.
Bui JD, Kimura B, Phillips MI . Losartan potassium, a nonpeptide antagonist of angiotensin II, chronically administered p.o. does not readily cross the blood-brain barrier. Eur J Pharmacol 1992; 219: 147–151.
Marshall FH, Clark SA, Michel AD, Barnes JC . Binding of angiotensin antagonists to rat liver and brain membranes measured ex vivo. Br J Pharmacol 1993; 109: 760–764.
Raghavendra V, Chopra K, Kulkarni SK . Comparative studies on the memory-enhancing actions of captopril and losartan in mice using inhibitory shock avoidance paradigm. Neuropeptides 2001; 35: 65–69.
Kjeldsen SE, Lyle PA, Kizer JR, Dahlof B, Devereux RB, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristianson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Snapinn SM, Harris KE, Wedel H . The effects of losartan compared to atenolol on stroke in patients with isolated systolic hypertension and left ventricular hypertrophy. The LIFE study. J Clin Hypertens (Greenwich) 2005; 7: 152–158.
Sink KM, Leng X, Williamson J, Kritchevsky SB, Yaffe K, Kuller L, Yasar S, Atkinson H, Robbins M, Psaty B, Goff DC Jr . Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the Cardiovascular Health Study. Arch Intern Med 2009; 169: 1195–1202.
Fogari R, Mugellini A, Zoppi A, Derosa G, Pasotti C, Fogari E, Preti P . Influence of losartan and atenolol on memory function in very elderly hypertensive patients. J Hum Hypertens 2003; 17: 781–785.
Polidori C, Ciccocioppo R, Nisato D, Cazaubon C, Massi M . Evaluation of the ability of irbesartan to cross the blood-brain barrier following acute intragastric treatment. Eur J Pharmacol 1998; 352: 15–21.
Tan J, Wang JM, Leenen FH . Inhibition of brain angiotensin-converting enzyme by peripheral administration of trandolapril versus lisinopril in Wistar rats. Am J Hypertens 2005; 18 (2 Pt 1): 158–164.
Braszko JJ, Karwowska-Polecka W, Halicka D, Gard PR . Captopril and enalapril improve cognition and depressed mood in hypertensive patients. J Basic Clin Physiol Pharmacol 2003; 14: 323–343.
Yamada K, Horita T, Takayama M, Takahashi S, Takaba K, Nagata Y, Suzuki N, Kanda T . Effect of a centrally active angiotensin converting enzyme inhibitor, perindopril, on cognitive performance in chronic cerebral hypo-perfusion rats. Brain Res 1421: 110–120.
Solfrizzi V, Scafato E, Frisardi V, Seripa D, Logroscino G, Kehoe PG, Imbimbo BP, Baldereschi M, Crepaldi G, Di Carlo A, Galluzzo L, Gandin C, Inzitari D, Maggi S, Pilotto A, Panza F . Angiotensin-converting enzyme inhibitors and incidence of mild cognitive impairment. The Italian Longitudinal Study on Aging. Age (Dordr) 2013; 35: 441–453.
Zuccala G, Onder G, Marzetti E, Lo Monaco MR, Cesari M, Cocchi A, Carbonin P, Bernabei R, Grp GS . Use of angiotensin-converting enzyme inhibitors and variations in cognitive performance among patients with heart failure. Eur Heart J 2005; 26: 226–233.
Yabuuchi F, Takahashi M, Aritake K, Fujimoto M, Ito H, Tsuzaki M, Akai T, Yamaguchi M, Hayashi S, Nishino Y, Brautigam M . Post-stroke treatment with imidapril reduces learning deficits with less formation of brain oedema in a stroke-prone substrain of spontaneously hypertensive rats. Fundam Clin Pharmacol 1999; 13: 475–483.
Leonetti G, Salvetti A . Effects of cilazapril and nitrendipine on blood pressure, mood, sleep, and cognitive function in elderly hypertensive patients: an Italian multicenter study. J Cardiovasc Pharmacol 1994; 24 ((Suppl 3)): S73–S77.
Starchina YA, Parfenov VA, Chazova IE, Sinitsyn VE, Pustovitova TS, Kolos IP, Ustyuzhanin DV . Cognitive function and the emotional state of stroke patients on antihypertensive therapy. Neurosci Behav Physiol 2007; 37: 13–17.
Iwanami J, Mogi M, Okamoto S, Gao XY, Li JM, Min LJ, Ide A, Tsukuda K, Iwai M, Horiuchi M . Pretreatment with eplerenone reduces stroke volume in mouse middle cerebral artery occlusion model. Eur J Pharmacol 2007; 566: 153–159.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yagi, S., Akaike, M., Ise, T. et al. Renin–angiotensin–aldosterone system has a pivotal role in cognitive impairment. Hypertens Res 36, 753–758 (2013). https://doi.org/10.1038/hr.2013.51
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2013.51
Keywords
This article is cited by
-
Risk of dementia in primary aldosteronism compared with essential hypertension: a nationwide cohort study
Alzheimer's Research & Therapy (2023)
-
Hypertension and cognitive dysfunction in elderly: blood pressure management for this global burden
BMC Cardiovascular Disorders (2016)
-
Chronic arterial hypertension impedes glioma growth: a multiparametric MRI study in the rat
Hypertension Research (2015)
-
Antihypertensive Therapies and Cognitive Function: a Review
Current Hypertension Reports (2015)
-
Serum concentration of eicosapentaenoic acid is associated with cognitive function in patients with coronary artery disease
Nutrition Journal (2014)