Abstract
The aim of this study was to determine whether hyperuricemia could predict future metabolic syndrome (MetS) in a large screened cohort of Japanese male and female subjects. We evaluated 5936 subjects (3144 male subjects, 2792 female subjects; mean age 48.7 years) who underwent health checkup programs in 2006 and 2010, who were MetS free in 2006. At baseline, hyperuricemia was detected in 927 male subjects (29.5%) and 276 female subjects (9.9%). Subjects with baseline hyperuricemia had significantly higher MetS prevalence in 2010 than those without (male subjects: 34.8 vs. 20.6%, P<0.0001; female subjects: 15.6 vs. 4.8%, P<0.0001). Compared with subjects in the first quintile of uric acid levels at baseline, the age-adjusted odds ratios (ORs) for MetS cumulative incidence among subjects in the third, fourth and fifth quintiles were, 1.8 (95% confidence interval (CI): 1.4–2.4: P<0.0001), 2.1 (95% CI: 1.6–2.8: P<0.0001) and 3.2 (95% CI: 2.4–4.1: P<0.0001), respectively, for male subjects and 2.4 (95% CI: 1.3–4.7: P=0.0075), 3.0 (95% CI: 1.6–5.7: P=0.0010) and 4.8 (95% CI: 2.6–8.8: P<0.0001), respectively for female subjects. Multivariable logistic analysis revealed that hyperuricemia was significantly associated with MetS cumulative incidence in male subjects (OR 1.5: 95% CI: 1.3–1.8, P<0.0001) and female (OR 2.0, 95% CI: 1.3–3.0, P<0.0001). In conclusion, hyperuricemia is a significant and independent predictor of MetS in Japanese male and female subjects. For both genders, MetS risk increases with increased serum uric acid levels.
Similar content being viewed by others
Introduction
Metabolic syndrome (MetS) is characterized by a combination of central obesity, atherogenic dyslipidemia (hypertriglyceridemia and low levels of high-density lipoprotein cholesterol (HDL-C)), hyperglycemia and elevated blood pressure (BP). The principal underlying pathophysiological abnormality of this syndrome is insulin resistance. MetS is a significant public health issue because it has become very common in both industrialized and developing countries and is associated with a higher risk of cardiovascular and all-cause mortality.1, 2
Hyperuricemia is also associated with the incidence of cardiovascular events.3, 4, 5, 6 Several studies have shown that hyperuricemia is correlated with classical cardiovascular risk factors7, 8, 9, 10 and MetS.11, 12, 13 Furthermore, three recent epidemiological studies found that hyperuricemia could be an independent predictor of MetS.14, 15, 16 However, the influence of gender on MetS incidence and serum uric acid (SUA) levels on increasing the risk of MetS differed among these studies.
Thus, the aim of the present study was to examine if hyperuricemia could predict future MetS in a large screened cohort of Japanese male and female subjects.
Methods
Subjects
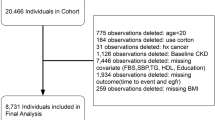
In 2006, 13 668 participants took part in a 1-day health checkup program run by the Okinawa General Health Association and 6919 participants were re-examined in 2010. Of the 6919 participants, we excluded 17 participants without available data of waist circumference (WC) in 2006 and/or 2010. Table 1A shows the numbers and prevalence of metabolic syndrome components, obesity, hypertension and diabetes mellitus according to the presence or absence of hyperuricemia before the exclusion of participants with metabolic syndrome in 2006. We also excluded 966 participants (820 male subjects, 146 female subjects) with MetS in 2006. As shown in Table 1A and Table 1B, in male subjects with hyperuricemia, almost half of high BP, hypertension, high blood glucose (BG) and diabetes mellitus were excluded. As a result, we included 5936 subjects (3144 male subjects, 2792 female subjects) in the present study. This study was approved by the ethics committee of the University Hospital of the Ryukyus.
The Okinawa General Health Association has managed the community-based health checkup program in Okinawa, Japan. Participants who visited the clinic of the Okinawa General Health Association completed a questionnaire that including details regarding family history of hypertension, diabetes mellitus, dyslipidemia, stroke and heart disease; lifestyle-related variables such as smoking and alcohol consumption; medical history; and current medications. Smoking habits were classified into one of three categories: non-smokers, ex-smokers or current smokers. Regarding alcohol consumption, participants were classified as non-drinkers, ex-drinkers or current drinkers of <1.5 cups, 1.5–3.0 cups or >3.0 cups of alcohol per day. One cup of sake contains 15% alcohol in 180 ml. The alcohol contents of other alcoholic beverages such as whisky, wine and beer were also calculated and 27 ml of alcohol was considered as one cup. Current medications included antihypertensive mediation use (yes/no), antidiabetic medication and/or insulin use (yes/no) and antidyslipidemic medication use (yes/no). The questionnaires were discussed during physical examinations, and a clinic physician interviewed the participants further.
All study subjects underwent a clinical examination, which included height, weight and WC measurements. WC was measured using a graduated tape at the umbilical level while subjects were standing. Body mass index (BMI) was calculated as body weight (kg) divided by height squared (m2). A well-trained nurse or doctor measured resting BP using a standard mercury sphygmomanometer while the subjects were sitting position after at least 5 min of rest.
All subjects fasted overnight before blood sampling. After fasting for at least 12 h, a venous blood sample was obtained. Levels of SUA, triglycerides, HDL-C and fasting blood glucose were determined for all subjects using an autoanalyzer (Hitachi Automatic Analyzer Model 7700, Hitachi High-Technologies, Tokyo, Japan) in the laboratory of the Okinawa General Health Association.
Definitions
There is no universally accepted definition of hyperuricemia. In the present study, we defined hyperuricemia as SUA levels of ⩾7.0 mg dl−1 in male subjects and ⩾6.0 mg dl−1 in female subjects. SUA levels were placed into five categories according to their quintile. For male subjects, UA1: ⩽5.4 mg dl−1; UA2: 5.5–6.0 mg dl−1; UA3: 6.1–6.6 mg dl−1; UA4: 6.7–7.3 mg dl−1; and UA5: ⩾7.4 mg dl−1. For female subjects, UA1: ⩽3.8 mg dl−1; UA2: 3.9–4.3 mg dl−1; UA3: 4.4–4.8 mg dl−1; UA4: 4.9–5.4 mg dl−1; and UA5: ⩾5.5 mg dl−1. Obesity was defined as a BMI ⩾25 kg m−2.
For this study, MetS was defined on the basis of criteria that were authorized by the Japanese Committee on the Criteria for MetS17 and commonly used in routine clinical practice in Japan. Participants with an increased WC (⩾85 cm in male subjects, ⩾90 cm in female subjects) and who had two or more of the following factors were diagnosed with MetS: high BP (systolic BP ⩾130 mm Hg and/or diastolic BP ⩾85 mm Hg and/or current use of antihypertensive medications); dyslipidemia (triglycerides levels ⩾150 mg dl−1 and/or HDL-C levels <40 mg dl−1 and/or current use of antidyslipidemic medications); and high blood glucose (high BG: fasting blood glucose levels ⩾110 mg dl−1 or current use of antidiabetic medications).
Statistical analysis
Results for continuous variables are given as means±s.d. and results for categorical variables are given as numbers and percentages (%). Independent t-tests were used to compare the results for continuous variables between groups with and without hyperuricemia. Results for categorical variables were compared by χ2-tests. Odds ratios (ORs) for the incidence of MetS were determined using multivariable logistic regression models. We used three logistic models to make adjustments (Model 1: adjusting for age; Model 2: adjusting for age, alcohol consumption, smoking status, presence or absence of increased WC, high BP, dyslipidemia, high BG, estimated glomerular filtration rate and presence or absence of medication use for hypertension and/or dyslipidemia and/or diabetes mellitus; and Model 3: adjusting for alcohol consumption, smoking status, presence or absence of increased WC, high BP, dyslipidemia, high BG, estimated glomerular filtration rate and presence or absence of medication use for hypertension and/or dyslipidemia and/or diabetes mellitus). The estimated glomerular filtration rate was calculated by means of the modified Cockcroft–Gault formula for Japanese populations.18 In subgroup analysis for age, we stratified the study subjects into tertile of age (Age T1: 20–42 years of age, Age T2: 43–52 years of age and Age T3: 65 year of age or older for male subjects; Age T1: 20–45 years of age, Age T2: 46–53 years of age and Age T3: 54 year of age or older for female subjects). Statistical analyses were performed using JMP 10.0 software (SAS Institute, Cary, NC, USA). A P-value of <0.05 was considered significant.
Results
At baseline (in 2006), the mean age of the study subjects was 48.7±9.8 years and 53.0% were male subjects. Hyperuricemia was detected in 927 male subjects (29.5%) and 276 female subjects (9.9%). The baseline characteristics of the study subjects with and without hyperuricemia are shown in Table 2. For both male and female subjects, those with hyperuricemia had significantly higher BMI, WC, diastolic BP, triglycerides levels and obesity prevalence.
With regard to MetS-components, female subjects with hyperuricemia had a significantly higher prevalence of increased WC, high BP, hypertension, dyslipidemia and high BG than those without. In addition, male subjects with hyperuricemia had a significantly higher prevalence of increased WC and dyslipidemia than those without. The prevalence of high BP, hypertension, high BG and diabetes mellitus was similar between male subjects with and without hyperuricemia.
In 2010, 944 of the 5936 subjects (779 male subjects, 165 female subjects) had progressed to MetS. Male subjects had a significantly higher cumulative incidence of MetS than female subjects (24.8 vs. 5.9%; P<0.0001). We found MetS incident in 323 male and 43 female subjects with hyperuricemia and 456 male and 122 female subjects without hyperuricemia, respectively. Subjects with hyperuricemia in 2006 had a significantly higher prevalence of MetS in 2010 than those without hyperuricemia in 2006 (male subjects: 34.8 vs. 20.6%; P<0.0001; female subjects: 15.6 vs. 4.8%; P<0.0001).
The baseline characteristics of the study subjects based on the occurrence of MetS in 2010 are shown in Table 3. For both male and female subjects, individuals who developed MetS had significantly higher measures for BMI, WC, diastolic BP, triglycerides, fasting blood glucose and uric acid (UA) than those who did not; in addition, individuals who developed MetS had a significantly higher prevalence of obesity, hyperuricemia, increased WC, dyslipidemia and high BG than those who did not.
Figure 1 shows the cumulative incidence of MetS according to SUA quintiles. The cumulative incidence of MetS significantly increased with increased SUA levels for both male and female subjects. The ORs (95% confidence intervals (CIs)) for the incidence of MetS compared with the lowest SUA quintile are shown in Table 4. We used two logistic models to make adjustments. With Model 1, the upper third, fourth and fifth SUA quintiles were significantly associated with MetS incidence and similar to those of crude ORs; the fifth quintile had the largest OR for both male and female subjects. With Model 2, the ORs also increased with increased SUA levels in male subjects. However, with Model 2, a significant association was only found with the upper fifth quintile for female subjects.
Cumulative incidence of metabolic syndrome based on serum uric acid (UA) quintiles. UA levels were placed into five categories: for male subjects: UA1: ⩽5.4 mg dl−1; UA2: 5.5–6.0 mg dl−1; UA3: 6.1–6.6 mg dl−1; UA4: 6.7–7.3 mg dl−1; and UA5: ⩾7.4 mg dl−1. For female subjects: UA1: ⩽3.8 mg dl−1; UA2: 3.9–4.3 mg dl−1; UA3: 4.4–4.8 mg dl−1; UA4: 4.9–5.4 mg dl−1; and UA5: ⩾5.5 mg dl−1. P<0.05 was considered significant.
The subgroup analyses were performed for examining influences of age on incidence of MetS. As shown in Table 5, Age T1 and Age T2 of male subjects showed significant associations of hyperuricemia with MetS incidence and the OR of Age T1 was larger than that of Age T2. On the other hand, in female subjects, the significant association between hyperuricemia and incidence of MetS was recognized only in Age T2 and the OR of female Age T2 was larger than that of all female subjects adjusted by Model 3.
We also examined the ORs (95% CIs) of hyperuricemia and the components of MetS compared with no hyperuricemia and the components of MetS for the cumulative incidence of MetS in male and female subjects. The age-adjusted logistic regression model (Model 1) revealed significant associations between hyperuricemia and the incidence of MetS in male subjects (OR 2.1, 95% CI: 1.7–2.4; P<0.0001) and female subjects (OR 3.3, 95% CI: 2.3–4.8; P<0.0001). After multivariable adjustment (Model 2), hyperuricemia also had significant associations with the incidence of MetS in both male subjects (OR 1.5, 95% CI: 1.3–1.8; P<0.0001) and female subjects (OR 2.0, 95% CI: 1.3–3.0; P<0.0001). Among the components of MetS, an increased WC had the highest OR for the incidence of MetS in both male and female subjects. With Model 1, ORs (95% CIs) were 5.5 (5.6–8.5; P<0.0001) for male subjects and 9.8 (7.0–13.7; P<0.0001) for female subjects. With Model 2, ORs (95% CIs) were 6.9 (5.6–8.5; P<0.0001) for male subjects and 12.7(8.8–18.3; P<0.0001) for female subjects, respectively.
Figure 2 shows the incidence rates of MetS components in subjects with incident MetS. The incidence of high BP was higher than those of WC, dyslipidemia and high BG, and the largest difference between subjects with hyperuricemia and those without hyperuricemia was recognized in the incidence of high BP in both male and female subjects.
Recently, the Japanese criteria for MetS were modified. The cut-off points for increased WC were 90 cm for male subjects and 80 cm for female subjects. There were proposed as being more predictive of cardiovascular disease.19 We applied these new criteria to our analyses. In 6902 participants who took part in the health checkups in both 2006 and 2010, 800 participants (503 male subjects, 297 female subjects) met the modified Japanese criteria for MetS at baseline. After exclusion of these 800 MetS subjects, 6102 subjects were reexamined. In 2010, 758 participants (506 male subjects, 252 female subjects) developed MetS. In Model 2, hyperuricemia was also a significantly associated with the incidence of MetS in male subjects (OR 1.9, 95% CI: 1.5–2.4; P<0.0001) and female subjects (OR 2.2, 95% CI: 1.0–4.9; P=0.0481).
Discussion
In this 4-year follow-up study, we showed that hyperuricemia was a significant and independent predictor of MetS in both male and female subjects. MetS risk increased with increased SUA levels. In female subjects, the risk increased with relatively lower SUA levels than those in male subjects.
There is limited information available regarding the prospective association between SUA levels and the MetS risk. Recently, three epidemiological studies showed close associations between UA levels and MetS incidence;14, 15, 16 however, some differences were found among these previous studies and the present study. The larger numbers of female subjects (2792) and higher cumulative incidence of MetS (16%) of our study compared with those of the three previous studies (1260–2109 female subjects and 4–14% of MetS incidence) might contribute to greater statistical power. The large study subjects of the current study could examine the association between UA quintiles and incident MetS during a shorter follow-up period (4 years) than that in the previous studies (⩾5 years) in both genders.
The influence of gender on MetS incidence and SUA levels on increasing the risk of MetS differed among the previous studies and the present study.14, 15, 16 For example, in the study by Hara et al.,15 a significant association between high SUA levels and the incidence of MetS was found in male subjects only. The higher incidence rates of MetS of the present study could explain the difference of findings. One possible reason for the differences in the incidence of MetS is that our study subjects had greater BMI values than those in the study by Hara et al.15
In the present study, female subjects had a MetS risk with relatively lower UA levels than male subjects (Table 4). Similar results were found in previous cross-sectional20 and prospective studies.16 The mechanism for these differences in associations with UA levels and MetS between male and female subjects is unclear. The interactions between SUA and sex hormones have not been thoroughly investigated, and understanding this relationship would help in understanding the stronger impact of hyperuricemia in female subjects.
The influence of age on hyperuricemia and MetS incidence differed between male and female subjects (Table 5). The large ORs were recognized in young male subjects and middle-aged female subjects, respectively. However, female subjects had smaller numbers of incident MetS than that of male subjects, and wide 95% CI of middle-aged female subjects might be caused by low statistical power. Further studies are needed to clarify the relationship among age, hyperuricemia and incidence of MetS in male and female subjects.
The finding of Figure 2 showed that high BP was a major contributor to the incidence of MetS in both genders. Furthermore, subjects with hyperuricemia had significantly higher incidence of high BP than that without hyperuricemia. Previous studies have reported a positive association between hyperuricemia and the risk for hypertension.7, 8, 10 Therefore, the association between hyperuricemia and incidence of hypertension may reflect on the incidence of MetS.
We also applied the new modified Japanese criteria for MetS.19 Our findings were similar to those based on the original Japanese criteria. Therefore, the cutoff point for WC in the Japanese criteria for MetS did not greatly influence the findings of the present study.
The precise biological mechanisms underlying the association between UA and development of MetS remain unclear. Nevertheless, it is recognized that visceral adiposity and insulin resistance may have an important role in the development of MetS. We found the positive association between SUA levels and the incidence of MetS (Figure 1) SUA levels are positively correlated with insulin resistance.21, 22 In addition, subjects with hyperuricemia had significantly greater WC and significantly higher prevalence of increased WC than those without hyperuricemia (Table 2). WC is a marker of visceral obesity and a greater WC correlates with a worsened state of insulin resistance:23 visceral adipose tissues secrete several adipocytokines such as adiponectin, leptin, tumor necrosis factor-1 and elevated production of the proinflammatory adipocytokines, which contribute to the development of insulin resistance.24 Moreover, the association between hyperuricemia and high BP described above could contribute to the incidence of MetS.
As shown in Table 2, we found the contradictory proportion of high BP and hypertension between male and female subjects. Before the exclusion of subjects with MetS in 2006, high BP and hypertension of male subjects with hyperuricemia were significantly higher than those of male subjects without hyperuricemia (Table 1A). Thus, we excluded large numbers of high BP and hypertension male subjects with hyperuricemia (Table 1B). As a result, a lower prevalence of hypertensive male individuals remained as study subjects than that of hypertensive female individuals. In Table 3, male subjects with MetS in 2010 contained significantly higher prevalence of hyperuricemia than that of male subjects without hyperuricemia. Therefore, the contradictory proportion of high BP and hypertension between male and female subjects in Table 3 can be similarly explained.
The present study had several limitations. First, we measured variables only once. Therefore, misclassifications may have occurred. Second, information on menopause was not available for our female subjects. Because of an interaction with sex hormones, SUA levels increase after menopause. Third, information on medication use of antihyperuricemic agents and diuretics was not available. No available data of antihyperuricemic agent use may underestimate the prevalence of hyperuricemia. Because the frequency of using diuretics as antihypertensives is low and the doses are low in Japan,25 the influence of diuretics on our results may be small. Fourth, although insulin resistance has a key role in MetS and hyperuricemia, we did not directly evaluate insulin resistance in the present study. Fifth, we also could not assess the dietary status of our study participants. Finally, the subjects in this study were self-selected and represented a relatively healthy population. The self-selected subjects may cause a selection bias.
In spite of these limitations, our study has strengths of a large sample size and sufficient numbers of subjects with developing MetS, which cause enough statistical power to determine the association between hyperuricemia and MetS incidence in both genders.
In conclusion, hyperuricemia is an independent predictor of MetS in male and female subjects. MetS risk increases with increased SUA levels, and in female subjects, the risk increases with relatively lower SUA levels than those in male subjects. Hyperuricemia is a useful marker that can easily predict future MetS in routine clinical practice.
References
Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT . The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002; 288: 2709–2716.
Ford ES . The metabolic syndrome and mortality from cardiovascular disease and all-causes: findings from the National Health and Nutrition Examination Survey II Mortality Study. Atherosclerosis 2004; 173: 309–314.
Fang J, Alderman MH . Serum uric acid and cardiovascular mortality: the NHANES I epidemiologic follow-up study, 1971-1992. National Health and Nutrition Examination Survey. JAMA 2000; 283: 2404–2410.
Alderman MH, Cohen H, Madhavan S . Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension 1999; 34: 144–150.
Verdecchia P, Schillaci G, Reboldi G, Santeusanio F, Porcellati C, Brunetti P . Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension 2000; 36: 1072–1078.
Hakoda M, Masunari N, Yamada M, Fujiwara S, Suzuki G, Kodama K, Kasagi F . Serum uric acid concentration as a risk factor for cardiovascular mortality: a longterm cohort study of atomic bomb survivors. J Rheumatol 2005; 32: 906–912.
Feig DI, Kang DH, Johnson RJ . Uric acid and cardiovascular risk. N Engl J Med 2008; 359: 1811–1821.
Johnson RJ, Kivlighn SD, Kim YG, Suga S, Fogo AB . Reappraisal of the pathogenesis and consequences of hyperuricemia in hypertension, cardiovascular disease, and renal disease. Am J Kidney Dis 1999; 33: 225–234.
Nagahama K, Iseki K, Inoue T, Touma T, Ikemiya Y, Takishita S . Hyperuricemia and cardiovascular risk factor clustering in a screened cohort in Okinawa, Japan. Hypertens Res 2004; 27: 227–233.
Nagahama K, Inoue T, Iseki K, Touma T, Kinjo K, Ohya Y, Takishita S . Hyperuricemia as a predictor of hypertension in a screened cohort in Okinawa, Japan. Hypertens Res 2004; 27: 835–841.
Choi HK, Ford ES . Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med 2007; 120: 442–447.
Tsouli SG, Liberopoulos EN, Mikhailidis DP, Athyros VG, Elisaf MS . Elevated serum uric acid levels in metabolic syndrome: an active component or an innocent bystander? Metabolism 2006; 55: 1293–1301.
Yoo TW, Sung KC, Shin HS, Kim BJ, Kim BS, Kang JH, Lee MH, Park JR, Kim H, Rhee EJ, Lee WY, Kim SW, Ryu SH, Keum DG . Relationship between serum uric acid concentration and insulin resistance and metabolic syndrome. Circ J 2005; 69: 928–933.
Sui X, Church TS, Meriwether RA, Lobelo F, Blair SN . Uric acid and the development of metabolic syndrome in women and men. Metabolism 2008; 57: 845–852.
Hara S, Tsuji H, Ohmoto Y, Amakawa K, Hsieh SD, Arase Y, Nakajima H . High serum uric acid level and low urine pH as predictors of metabolic syndrome: a retrospective cohort study in a Japanese urban population. Metabolism 2011; 61: 281–288.
Yang T, Chu CH, Bai CH, You SL, Chou YC, Chou WY, Chien KL, Hwang LC, Su TC, Tseng CH, Sun CA . Uric acid level as a risk marker for metabolic syndrome: a Chinese cohort study. Atherosclerosis 2012; 220: 525–531.
Definition and the diagnostic standard for metabolic syndrome—Committee to Evaluate Diagnostic Standards for Metabolic Syndrome. Nippon Naika Gakkai Zassi 2005; 94: 749–809 [in Japanese].
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A . Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992.
Hata J, Doi Y, Ninomiya T, Tanizaki Y, Yonemoto K, Fukuhara M, Kubo M, Kitazono T, Iida M, Kiyohara Y . The effect of metabolic syndrome defined by various criteria on the development of ischemic stroke subtypes in a general Japanese population. Atherosclerosis 2010; 210: 249–255.
Chiou WK, Wang MH, Huang DH, Chiu HT, Lee YJ, Lin JD . The relationship between serum uric acid level and metabolic syndrome: differences by sex and age in Taiwanese. J Epidemol 2010; 20: 219–224.
Vuorinen-Markkola H, Yki-Jarvinen H . Hyperuricemia and insulin resistance. J Clin Endocrinol Metab 1994; 78: 25–29.
Modan M, Halkin H, Karasik A, Lusky A . Elevated serum uric acid—a facet of hyperuinulinaemina. Diabetologia 1987; 30: 713–718.
Reaven GM . The metabolic syndrome: time to get off the merry-go-round? J Intern Med 2011; 269: 127–136.
Bruce KD, Byrne CD . The metabolic syndrome: common origins of a multifactorial disorder. Postgrad Med J 2009; 85: 6146–6121.
Yamamoto Y, Sonoyama K, Matsubara K, Furuse M, Yatsuhashi T, Hamada T, Ogino K, Igawa O, Hisatome I, Shigemasa C . The status of hypertension management in Japan in 2000. Hypertens Res 2002; 25: 717–725.
Acknowledgements
The authors are grateful for the cooperation of the staff of the Okinawa General Health Maintenance Association.
Author contributions
KK conceived data collection, TI, KK and AI provided valuable assistance and YO co-wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
All authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Nagahama, K., Inoue, T., Kohagura, K. et al. Hyperuricemia predicts future metabolic syndrome: a 4-year follow-up study of a large screened cohort in Okinawa, Japan. Hypertens Res 37, 232–238 (2014). https://doi.org/10.1038/hr.2013.137
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2013.137
Keywords
This article is cited by
-
Prevalence of Diabetes in Patients with Hyperuricemia and Gout: A Systematic Review and Meta-analysis
Current Diabetes Reports (2023)
-
The bidirectional relationship between metabolic syndrome and hyperuricemia in China: A longitudinal study from CHARLS
Endocrine (2022)
-
Association between baseline and changes in serum uric acid and incident metabolic syndrome: a nation-wide cohort study and updated meta-analysis
Nutrition & Metabolism (2021)
-
Association between serum uric acid and metabolic syndrome: a cross-sectional study in Bangladeshi adults
Scientific Reports (2020)
-
Prevalence and risk factors associated with hyperuricemia among working population at high altitudes: a cross-sectional study in Western China
Clinical Rheumatology (2019)