Abstract
Epoxyeicosatrienoic acids (EETs), cytochrome P450-derived metabolites of arachidonic acid, have been reported to increase intracellular calcium concentration in aortic vascular smooth muscle cells (SMCs). As EETs are labile, we synthesized a new stable urea EET analog with agonist and soluble epoxide hydrolase (sEH) inhibitor properties. We refer to this analog, 12-(3-hexylureido)dodec-8-enoic acid, as 8-HUDE. Measuring tension of vascular rings, intracellular calcium signaling by confocal laser scanning microscopy and gene expression by reverse-transcription-PCR and western blots, we examined the effects of 8-HUDE on pulmonary vascular tone and calcium signaling in rat pulmonary artery (PA) SMCs (PASMCs). 8-HUDE increased the tension of rat PAs to 145% baseline, whereas it had no effect on the tension of mesenteric arteries (MAs). The 8-HUDE-induced increase in vascular tone was abolished by removal of extracellular Ca2+ or by pretreatment with either La3+ or SKF96365, which are inhibitors of canonical transient receptor potential channels (TRPCs). Furthermore, 8-HUDE-evoked increases in [Ca2+]i in PASMCs could be blunted by inhibition of TRPC with SKF96365, removal of extracellular calcium or depletion of intracellular calcium stores with caffeine, cyclopiazonic acid or 2-aminoethoxydiphenyl borate, but not by the voltage-activated calcium channel blocker nifedipine. In addition to immediate effects on calcium signaling, 8-HUDE upregulated the expression of TRPC1 and TRPC6 at both mRNA and protein levels in rat PASMCs, whereas it suppressed the expression of sEH. Our observations suggest that 8-HUDE increases PA vascular tone through increased release of calcium from intracellular stores, enhanced [Ca2+]i influx in PASMCs through store-operated Ca2+ channels and modulated the expression of TRPC and sEH proteins in a proconstrictive manner.
Similar content being viewed by others
Introduction
Arachidonic acid is catalyzed by cytochrome P450 epoxygenases to four regioisomeric epoxyeicosatrienoic acids (EETs): 5,6-, 8,9-, 11,12- and 14,15-EET. EETs are potent, biologically important dilators in a number of vascular beds, including renal, cerebral and coronary.1, 2, 3, 4 In fact, EETs have been identified as potential endothelial-derived hyperpolarizing factors in the renal and coronary circulations.5, 6, 7, 8, 9 In contrast, we have reported that EETs constrict rabbit pulmonary arteries (PAs) in a concentration-dependent manner.10 These lipids also exhibit anti-inflammatory properties.5, 6, 8, 9
Some reports suggest that EETs increase [Ca2+]i in vascular smooth muscle cells (SMCs) in a manner that depends on extracellular Ca2+ and voltage-dependent (L-type) Ca2+ channels.11 EETs-induced increase in [Ca2+]i could promote vasorelaxation through activation of calcium-activated, large-conductance potassium channels. On the other hand, intracellular calcium increases might also have proconstrictive effects and, therefore, may modulate EET-induced vasorelaxation. To our knowledge, the effects of EETs on intracellular calcium concentrations in pulmonary vascular SMCs are unknown.
In common with most eicosanoids, EETs are chemically and metabolically labile, which is a property that limits potential therapeutic applications. Moreover, EETs act as substrates for soluble epoxide hydrolase (sEH) enzymes; metabolism of EETs by sEH into the corresponding dihydroxyeicosatrienoic acids leads to partial or complete loss of activity.12, 13, 14 Owing to the cardiovascular and renal protective effects by EETs, inhibiting their metabolism by sEH may be a potential therapeutic target in diseases such as hypertension and inflammation.15 Such treatment would benefit a host by combining the effects of 8,9-EET while simultaneously suppressing their degradation.
The question we raised is whether a stable EET analog that inhibits sEH would have effects on PA (as opposed to mesenteric artery (MA)) tension, and if so, what signaling pathways would mediate these changes. In this study, we show that a stable EET urea agonist/sEH inhibitor, 12-(3-hexylureido)dodec-8-enoic acid (8-HUDE) regulates vasoconstriction of PAs through enhanced release of calcium from intracellular stores and activation of canonical transient receptor potential channels (TRPCs).
Methods
Materials
Both 8-HUDE and 8,9-EET were synthesized in the laboratory of Dr JR Falck; SKF96365 and nifedipine were purchased from Biomol (Plymouth Meeting, PA, USA). Caffeine, La3+ and cyclopiazonic acid (CPA) were obtained from Sigma-Aldrich (St Louis, MO, USA). 2-Aminoethoxydiphenyl borate (2-APB) and 12-(3-adamantan-1-yl-ureido) dodecanoic acid (AUDA) were purchased from Cayman Chemical (Ann Arbor, MI, USA). Anti-TRPC1 and anti-TRPC6 antibodies were purchased from Sigma-Aldrich and from Alomone Labs (Jerusalem, Israel), respectively. β-Actin and sEH antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Enhanced chemiluminescence reagents were obtained from Amersham International (Amersham, UK). Fluo 3-acetoxymethyl ester was purchased from Molecular Probes (Eugene, OR, USA). The reverse-transcription-PCR kit was purchased from Invitrogen (Carlsbad, CA, USA). All other chemicals were obtained from common commercial sources.
Culture and protocol of PASMCs
Adult female/male Wistar rats were obtained from the Experimental Animal Center of Harbin Medical University, which is fully accredited by an Institutional Animal Care and Use Committee. The primary culture of PASMCs was prepared as published previously.16 Cells were cultured with DMEM (Dulbecco's modified eagle's medium), which contained 20% fetal bovine serum, 1% penicillin and streptomycin in an incubator chamber at 37 °C under 5% CO2. The purity of primary cultures was evaluated by specific monoclonal antibodies raised against smooth muscle α-actin (Boehringer, Mannheim, Germany). Second- and third-passage cells were used for further experimentation.
Tension studies of PA and MA rings
Both PAs and MAs were carefully dissected from adult rats, and then cut into small pieces ∼1 mm in diameter and 2–3 mm in length, with the connective tissue removed.17 After tungsten wires were inserted into freshly isolated PA and MA rings, the rings were mounted in the tension-detecting device, and submerged in Krebs solution (in mM: NaCl 116, KCl 4.2, CaCl2 2.5, NaH2PO4 1.6, MgSO4 1.2, NaHCO3 22 and D-glucose 11) bubbled with 95% oxygen and 5% CO2. Both PA and MA rings were equilibrated with 0.3 g passive tension for 1–2 h. Either 8,9-EET or 8-HUDE was added to the bath at concentrations ranging from 10−8 to 10−5 mol l−1 at 10-min intervals. In some experiments as indicated, rings were pretreated for 30 min with SKF96365 (50 μM), La3+ (30 μM) or nifedipine (1 μM) before addition of the analog. In other experiments, calcium was removed from the external solution by exchange of the bath for a Tyrode's solution with the following constituents (in mM: NaCl 126, KCl 5, HEPES 10.0, NaH2PO4 0.3, MgCl2 3.0, glucose 10, EGTA (ethylene glycol tetraacetic acid) 1.0). All rings were contracted with 1.0 μM phenylephrine to ensure tissue vitality at the end of the experiment. Rings that did not exhibit a doubling of tension to phenylephrine were excluded from the analysis. Data acquisition and analysis were facilitated by CODAS software DataQ Instruments, Inc. (Cleveland/Akron, OH, USA).
[Ca2+]i FI measurement
[Ca2+]i in PASMCs was measured using the membrane-permeable acetoxymethyl ester form of the Ca2+-sensitive fluorescent dye fluo 3 and fluorescent microscopy as described previously.18 The fluorescence intensity (FI) of [Ca2+]i was detected and excited at 488 nm, while collecting emitted light at 508 nm by confocal laser scanning microscopy. After a baseline recording, cells were exposed to 8-HUDE (10−5 M) or 8,9-EET (10−5 M) and data acquired for 600 s at 5- or 20-s intervals. To examine the contribution of specific channels to [Ca2+]i elevation, some groups of cells were pretreated with channel blockers or depleting agents including SKF96365, caffeine and 2-APB for 20 min before 8-HUDE or 8,9-EET challenge. Image analysis was performed offline using Fluoview-FV300 (Olympus, Japan) to select cell regions from which FI was extracted and further analyzed using Excel Microsoft Corporation (Redmond, WA, USA) and Origin Version 7.5 software (OriginLab Corporation, Northampton, UK). [Ca2+]i changes over time were assessed by normalizing to initial FI (FI0).
Reverse-transcriptase-PCR
Total RNA was extracted using the Trizol reagent from cultured PASMCs according to the manufacturer's instructions and detected by ultraviolet spectrophotometry. Extracted total RNAs were reverse-transcribed using the Superscript first-strand cDNA synthesis kit (Invitrogen). cDNA samples were amplified in a DNA thermal cycler (Thermo Scientific, Waltham, MD, USA). Gene-specific primers were designed from their own coding regions as follows: TRPC1 (GenBank accession no. DQ-839447: forward: 5′-CTCTTGACAAACGAGGACTACTAG-3′, reverse: 5′-GTCTTCCAACCCTTCATACCAC-3′, fragment size: 466 bp); TRPC6 (GenBank accession no. AB-051213: forward: 5′-AAAGATATCTTCAAATTCATG-GTC-3′, reverse: 5′-CACGTCCGCATCATCCTCAATTTC-3′, fragment size: 323 bp); sEH (GenBank accession no. NM-022936: forward: 5′-GCGGGCTTTCGTGTTCTA-3′, reverse: 5′-TCAGGATTTGGTGGCATT-3′, fragment size: 254 bp) and β-actin (GenBank accession no. NM_031144.2: forward: 5′-ACTATCGGCAATGAGCG-3′, reverse: 5′-GAGCCAGGGCAGTAATCT-3′, fragment size: 220 bp). Reaction products were separated by electrophoresis on 1% agarose gel stained with ethidium bromide. Images were obtained and band intensities analyzed using gel imaging analysis system (Alpha Innotech, San Leandro, CA, USA).
Protein preparation/western blotting
When cells reached 80% confluence, they were placed in the DMEM medium without serum for 24 h. Thereafter, cells were treated with 8-HUDE or 8,9-EET in DMEM, which contained 5% fetal bovine serum and treated with media alone as control. Vehicle, native 8,9-EET or 8-HUDE was added every 6 h. After 24 or 48 h, cells were washed with cold phosphate-buffered saline three times, and then lysed with a lysis buffer (Tris 50 mM, pH 7.4, NaCl 150 mM, TritonX-100 1%, EDTA 1 mM and PMSF 2 mM) on ice. The lysates were sonicated for 1 min and then centrifuged at 12 000 r.p.m. for 10 min at 4 °C. The protein concentration of the supernatants was determined using the Bio-Rad protein assay kit (Bio-Rad Laboratories, Inc., Berkeley, CA, USA). Samples containing 20 μg total protein were heated with SDS-PAGE sample buffer for 5 min at 95 °C and separated by 8 or 12% SDS-PAGE gels. After separation, proteins were transferred to nitrocellulose membrane. Membranes were blocked for 1 h at room temperature with Tris-buffered saline buffer (20 mM Tris, 150 mM NaCl, pH 7.6 Tween 20 0.1%) containing 5% nonfat dry milk. Membranes were incubated overnight at 4 °C in Tris-buffered saline Tween 20 containing bovine serum albumin and primary antibody, such as anti-TRPC1 (1:200 dilution) or anti-TRPC6 (1:200 dilution), anti-β-actin (1:5000 dilution) and anti-sEH (1:200 dilution). Imaging and semi-quantitation of the density of bands of interest were performed according to our previously published methods.19
Statistical analysis
Experimental data are expressed as means±s.e.m. Statistical analysis was performed with one-way ANOVA (analysis of variance), followed by Dunnett's test where appropriate. Differences were considered to be significant at P<0.05.
Results
Chemical structure of 8,9-EET and analog
The chemical structures of 8,9-EET and 8-HUDE appear in Figure 1. The analog is partially saturated (Figure 1b), which obviates metabolism by cyclooxygenase and lipoxygenase (LOX), as well as auto-oxidation. 8-HUDE also has sEH inhibitor activity7 as measured using recombinant human epoxide hydrolase.
Effects of 8-HUDE on the tension of PA and MA rings
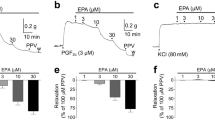
To examine the role of 8-HUDE on PA and MA tone, a concentration-response curve of the analog on the tension of isolated PA and MA rings was determined. 8-HUDE at a concentration of 10−5 M increased PA tension to ∼144% baseline (Figures 2b, d and e; n=10; P<0.05). The concentration-dependent constriction of PA rings to 8-HUDE was not different from that of native 8,9-EET (Figure 2f). In rat MA rings, treatment with 8-HUDE from 10−8 to 10−5M had no effect on tension (n=6) (Figures 2a, c and e). Therefore, PAs, but not MAs, increase tension in response to this analog.
(a, b) A representative concentration response relationship to 8-HUDE (10−8 to 10−5 M) in MA and PA rings. (c, d) Representative recording showing the effect of the of 10−8 M to 10−5 M analog on MA rings and PA rings pre-contracted with 10−6 M PE in (n=6 each). (e) Concentration-dependent relation in MA and PA rings induced by 8-HUDE (10−8 to 10−5 M) (n=10; *P<0.05, **P<0.001). (f) Average response of PA rings to 8,9-EET and 8-HUDE (n=10; *P<0.05 8,9-EET vs. vehicle, #P<0.05 8-HUDE vs. vehicle). (g) Average response of PA rings to the analog without extracellular Ca2+ (n=6; *P<0.05). (h) Effects of La3+ (30 μM) and SKF96365 (50 μM) on the response of PA rings to the analog (n=6; *P<0.05 La3+ vs. control, #P<0.05, SKF96365 vs. control; each value represents mean±s.e.m.). (i) Effects of nifedipine (1 μmol l−1), an inhibitor of voltage-dependent (L-type) Ca2+ channels on the change in tension of PA rings to the analog (n=6). There was no effect of nifedipine on tension in response to 8-HUDE. Analog means 8-HUDE herein. EET, epoxyeicosatrienoic acid; 8-HUDE, 12-(3-hexylureido)dodec-8-enoic acid; MA, mesenteric artery; PA, pulmonary artery; PE, phenylephrine.
8-HUDE-induced PA vasoconstriction depends on extracellular Ca2+ and TRPC
In isolated PA rings, removal of extracellular Ca2+ (Tyrode's solution with 1 mM EGTA) effectively eliminated the active tension induced by the analog (tension increased to only 103±2% baseline; n=6; Figure 2g). We next examined the role of store-operated Ca2+ influx on 8-HUDE-induced increases in PA tension. We used two separate inhibitors of TRPCs (La3+ 30 μmol l−1 or SKF96365 50 μmol l−1) and found that analog-evoked increases in the tension of PA rings were strongly blocked by these agents (Figure 2h). In contrast, an inhibitor of voltage-dependent calcium channels, nifedipine (1 μmol l−1) had no effect on increases in tension to 8-HUDE (tension increased to 146% baseline; n=6; Figure 2i). These data suggest that TRPCs, but not L-type Ca2+ channels are involved in pulmonary vasoconstriction induced by 8-HUDE.
The effect of 8-HUDE on FI of [Ca2+]i
[Ca2+]i FI is increased by 8,9-EET in PASMCs in a time- and extracellular calcium-dependent manner (Figures 3a–c). 8-HUDE also increased the FI of [Ca2+]i to a maximum of ∼150% baseline levels over 500 s in the presence of extracellular Ca2+. When extracellular calcium was chelated with EGTA (Tyrode's solution), 8-HUDE-induced increases in [Ca2+]i were roughly half that of cells studied in the presence of extracellular Ca2+ (Figures 3d–f). These data indicate that extracellular Ca2+ influx contributes to the increase in intracellular Ca2+ induced by 8-HUDE and native 8,9-EET.
Effects of 8,9-EET and 8-HUDE on intracellular Ca2+ FI in PASMCs in the presence and absence of extracellular Ca2+. (a, d) Time course depicting the rate of change in Ca2+ induced by 8,9-EET and 8-HUDE. (b) Ca2+ changes induced by vehicle (bottom line, 6.8 μl, n=21 from 3 animals) or 8,9-EET (10 μM) in the presence (top line, n=25 from 4 animals) and absence (middle line, n=30 from 4 animals) of extracellular Ca2+. (c) Increase of the AUC of the Ca2+ response induced by 8,9-EET in the presence or absence of extracellular Ca2+ was different (*P<0.05, two-tailed unpaired t-test, n=number of cells imaged). (e, f) Average trace and AUC of 8-HUDE (10 μM) induced Ca2+ change in the presence (top line, n=19 from 4 animals) and absence (middle line, n=24 from 4 animals) of extracellular Ca2+ (*P<0.05, two-tailed unpaired t-test, n=number of cells imaged). Analog means 8-HUDE herein. AUC, area under the curve; EET, epoxyeicosatrienoic acid; 8-HUDE, 12-(3-hexylureido)dodec-8-enoic acid; FI, fluorescence intensity; PASMC, pulmonary artery smooth muscle cell.
Contribution of intracellular Ca2+ channels to the increase in Ca2+ FI induced by 8-HUDE, and assessment of the role of CCE in analog-evoked increases in calcium in PASMCs
Ryanodine receptors are activated by caffeine, which then depletes ryanodine receptor-operated Ca2+ stores.20 Ca2+ released from the inositol trisphosphate (IP3) receptor is reported to be blocked by 2-APB.21 CPA depletes Ca2+stores from the sarcoplasmic reticulum. In this study, PASMCs were incubated with caffeine (5 mM), 2-APB (75 μM) or CPA (10 μM) for 20 min in Tyrode's solution with Ca2+ chelated (in mM: NaCl 126, KCl 5, HEPES 10, NaH2PO4 0.3, MgCl2 3.0, glucose 10, EGTA 1.0) to study the source of calcium increases induced by 8-HUDE. In the presence of 2-APB, [Ca2+]i increased to a maximum of 116% baseline over 500 s (Figure 4a), and to 108% baseline over 500 s in the presence of caffeine (Figure 4a). CPA effectively eliminated 8-HUDE-induced increases in [Ca2+]i. These observations suggest that both ryanodine and IP3 receptors participate in 8-HUDE-induced Ca2+signaling.
Contribution of intracellular Ca2+ stores to the alteration of intracellular Ca2+ FI induced by 8-HUDE in PASMCs: restoration of extracellular Ca2+ after store depletion with CPA. (a) Average response to 8-HUDE (10 μM, n=15) and after incubation with 2-APB (75 μM, n=15) for 10 min or caffeine (5 mM, n=15) or CPA (10 μM, n=15) in Ca2+-free Tyrode's solution. (b) AUC of the Ca2+ response related to different treatments in Ca2+-free Tyrode's solution (*P<0.05, two-tailed unpaired t-test, n=number of cells imaged). (c, d) Representative traces from PASMCs first in 0 [Ca2+] initially for 20 min (this phase not shown). Vertical arrows labeled ‘a’ and ‘c’ indicate addition of CPA and nifedipine to the bath. In both cases, there is a self-limited spike in calcium consistent with CPA-induced release from intracellular stores. At vertical arrows ‘b’ and ‘d,’ the bath was changed to one containing 2.5 mM Ca2+, as well as CPA and nifedipine. Vehicle (‘b’) or 8-HUDE (‘d’) was added simultaneously to the restoration of external calcium. Repletion of calcium resulted in a larger influx of calcium in 8-HUDE- than in vehicle-treated (control) cells. (e) Bar graph illustrating the AUC of the increase in [Ca2+]i FI/FI0 in the second-phase rise from panels c and d after different treatments and replenishment of external calcium (*P<0.05). Analog means 8-HUDE herein. AUC, area under the curve; CPA, cyclopiazonic acid, 8-HUDE, 12-(3-hexylureido)dodec-8-enoic acid; FI, fluorescence intensity; FI0, initial FI; PASMC, pulmonary artery smooth muscle cell; 2-APB, 2-aminoethoxydiphenyl borate.
In the next set of experiments, we studied the role of capacitative calcium entry (CCE) in PASMC calcium responses to 8-HUDE. CCE was assessed by restoration of extracellular calcium to PASMCs perfused for 20 min with Ca2+-free modified Krebs solution containing 0.5 mmol l−1 EGTA in external solutions to chelate any residual Ca2+, 10 μM CPA to deplete intracellular calcium stores and 5 μM nifedipine to prevent calcium entry through voltage-dependent calcium channels. [Ca2+]i was measured at 5-s intervals before and after restoration of extracellular [Ca2+]i to 2.5 mmol l−1 (by perfusion with normal modified Krebs solution) in the continued presence of nifedipine and CPA. As shown in Figures 4c and d, CPA resulted in a self-limited calcium transient consistent with the release from intracellular stores. Subsequent restoration of extracellular Ca2+ induced a second increase in [Ca2+]i FI/FI0 to 1.68±0.02 (n=22 cells in 5 experiments) in vehicle-treated cells. In 8-HUDE-treated PASMCs, restoration of extracellular Ca2+ resulted in a greater increase in [Ca2+]i (to 2.14±0.04, respectively, n=20 cells in 6 experiments).
The role of TRPCs in Ca2+ signaling induced by 8-HUDE
We next used SKF96365 (50 μM) to examine the contribution of TRPCs to Ca2+ signaling induced by 8-HUDE. In the presence of SKF96365, 8-HUDE-induced increases in [Ca2+]i were decreased to a maximum of ∼112% baseline over 500 s (compared with Figure 5a). Pretreatment with SKF96365 also reduced the influx of extracellular Ca2+ as estimated by the area under the curve as shown in Figure 5b. To determine whether voltage-dependent calcium channels were involved in 8-HUDE-stimulated entry of calcium, we preincubated with nifedipine (5 μM for 20 min). In contrast to treatment with SKF96365, nifedipine had no effect on [Ca2+]i (Figures 5a and b).
Contribution of TRPCs and not VDCCs to increases in intracellular Ca2+ induced by 8-HUDE. (a) Average [Ca2+]i response in the absence (n=17) and presence (n=16) of SKF or nifedipine (50 or 5 μM, respectively, pretreatment for 20 min). (b) SKF decreased the AUC of the Ca2+ response induced by 8-HUDE, whereas nifedipine had no effect on the AUC of the Ca2+ response (*P<0.05, two-tailed unpaired t-test, n=number of cells imaged). Analog means 8-HUDE herein. AUC, area under the curve; 8-HUDE, 12-(3-hexylureido)dodec-8-enoic acid; TRPC, canonical transient receptor potential channel; VDCC, voltage-dependent calcium channel.
The effect of sEH signaling on [Ca2+]i in PASMCs induced by 8-HUDE
We used 1 μM AUDA to examine the contribution of sEH to 8-HUDE-evoked changes in [Ca2+]i. AUDA alone had no effect on intracellular calcium. The increase in calcium was not different in PASMCs treated with 8,9-EET, 8-HUDE or 8-HUDE after pretreatment with AUDA (Figure 6). These data suggest that EET-like function, and not sEH inhibition, is responsible for 8-HUDE-associated calcium signaling in the time frame of these experiments.
The effect of sEH signaling on [Ca2+]i in PASMCs induced by 8-HUDE. (a) Representative images of the time course of Ca2+ changes induced by 8-HUDE in PASMCs pretreated with AUDA for 5 min. (b) Average trace of Ca2+ changes induced by 8,9-EET (bottom line, 10 μM, n=25 from 4 animals) and 8-HUDE (10 μM) in the presence (top line, n=22 from 4 animals) and absence (middle line, n=19 from 4 animals) of AUDA. (c) AUC of the Ca2+ response induced by 8-HUDE alone, 8,9 EET alone, AUDA alone or 8-HUDE in cells pretreated with AUDA. Analog means 8-HUDE herein. AUC, area under the curve; AUDA, 12-(3-adamantan-1-yl-ureido) dodecanoic acid; EET, epoxyeicosatrienoic acid; 8-HUDE, 12-(3-hexylureido)dodec-8-enoic acid; PASMC, pulmonary artery smooth muscle cell; sEH, soluble epoxide hydrolase. *P<0.05, two-tailed unpaired t test, n=number of cells imaged.
Time-dependent changes in TRPC1 and TRPC6 expression in PASMCs treated with 8-HUDE
To determine the effect of 8-HUDE on the expression of TRPCs, we performed reverse-transcription-PCR and western blot analyses on total RNA and protein extracted from PASMCs at 6, 12, 24 and 48 h after the addition of vehicle or analog. Both mRNA and protein levels of TRPC1 and TRPC6 increased after 24 h of exposure to 8-HUDE (Figures 7a–d). We then tested the capacity of native 8,9-EET to increase the expression of mRNA or protein for TRPC1 and TRPC6 at 24 h (the peak response for 8-HUDE). These data show that 8-HUDE increased TRPC1 mRNA and protein to greater levels than did native 8,9-EET, whereas the induction of TRPC6 was identical for native 8,9-EET and 8-HUDE (Figures 7e–h).
(a–d) Time course of the expression of TRPC1 and TRPC6 mRNA and protein in cultured rat PASMCs exposed to 8-HUDE. TRPC1 (panels a and b) and TRPC6 (panels c and d) mRNA and protein expressions were analyzed separately in PASMCs cultured in the presence of 8-HUDE or vehicle for different times as indicated. Bar graphs show means±s.e.m. data for TRPC1 (panels a and b) and TRPC6 (panels c and d) expressions normalized to β-actin mRNA and protein (*P<0. 05, **P<0.001). (e–h) Effect of 8-HUDE or native 8,9-EET on TRPC mRNA and protein expression. (Panels e and g) PCR-amplified products were displayed in agarose gels stained with ethidium bromide for TRPC1 and TRPC6 and β-actin. Both native 8,9-EET and 8-HUDE increased the expression of TRPC1 and TRPC6 mRNA in PASMCs. (Panels f and h) TRPC1 and TRPC6 protein expression in PASMCs treated for 24 h with 8-HUDE or 8,9-EET. All values are denoted as mean±s.e.m. from three or more separate experiments (*P<0.05 compared with the control group; #P<0.05 compared with the 8-HUDE group). Both 8,9-EET and 8-HUDE increased expression of the TRPC1/6 protein. Analog means 8-HUDE herein. EET, epoxyeicosatrienoic acid; 8-HUDE, 12-(3-hexylureido)dodec-8-enoic acid; PASMC, pulmonary artery smooth muscle cell; TRPC, canonical transient receptor potential channel.
The effect of 8-HUDE on sEH expression in PASMCs
To evaluate a distinct role of 8-HUDE as an inhibitor of sEH expression, we used reverse-transcription-PCR and western blot assays. sEH expression at mRNA levels started declining 24 h after treatment with 10−7 M 8-HUDE, and protein levels in cells exposed to 10−5 M 8-HUDE (Figures 8a and b). Interestingly, inhibition of sEH activity with 1 μM AUDA also inhibited the expression of sEH, particularly at the level of mRNA (Figures 8c and d).
Effect of 8-HUDE on sEH mRNA and protein expression. (a, b) sEH mRNA and protein expression were analyzed separately in PASMCs cultured in the presence of 8-HUDE from 10−8 to 10−5 M for 24 h (*P<0.05, n=6). (c, d) AUDA (1 μM), an accepted sEH inhibitor, was also tested for the potential to inhibit sEH mRNA and protein expression (*P<0.05, n=6). Analog means 8-HUDE herein. AUDA, 12-(3-adamantan-1-yl-ureido) dodecanoic acid; 8-HUDE, 12-(3-hexylureido)dodec-8-enoic acid; PASMC, pulmonary artery smooth muscle cell; sEH, soluble epoxide hydrolase.
Discussion
In this study, we first noted that the tension of PA, but not of MA, rings is enhanced by both native 8,9-EET and a stable EET analog with sEH inhibitory properties (8-HUDE). In the absence of external Ca2+, the proconstrictive properties of 8-HUDE in PAs were substantially diminished. Next, we examined the calcium influx pathways activated by 8-HUDE to induce PA vasoconstriction. After blocking TRPCs with La3+ or SKF96365, the capacity of 8-HUDE to increase PA tension was blunted. In contrast, after inhibition of L-type Ca2+ channels with nifedipine, PAs still constricted to the analog, supporting the role of TRPCs over voltage-gated calcium channels in 8-HUDE-induced PA vasoconstriction. This conclusion was reinforced by experiments in which 8-HUDE-induced increases in [Ca2+]i in PASMCs were blocked by the TRPC inhibitor SKF96365, as well as by inhibition of IP3 and ryanodine receptors. Restoration of [Ca2+]i in PASMCs first depleted by treatment with CPA and chelation of external calcium in the presence of nifedipine to block voltage-dependent calcium channels were enhanced by 8-HUDE on return of cells to calcium-containing external solution (Figure 4). Taken together, these data are most consistent with the interpretation that 8-HUDE causes PA vasoconstriction through enhanced CCE in PASMCs. We speculate that the analog evokes (1) Ca2+ release from intracellular Ca2+ stores by the IP3 receptor and ryanodine receptors and (2) calcium influx by store-operated Ca2+ channels, specifically TRPC1 and TRPC6. In addition to acute effects on intracellular calcium, subacute exposure of PASMCs to 8-HUDE elevated the expression of TRPC1 and TRPC6, consistent with previous reports that EETs increase the expression of TRPC6 channels in rat PASMCs.22 Finally, 8-HUDE decreased the expression of sEH in PASMCs, an effect that would be anticipated to result in sustained increases in tension in PAs over several hours to days.
Epoxyeicosatrienoic acids, cytochrome P450 epoxygenase metabolites of arachidonic acid, have potent vasoactive effects in a number of vascular beds and are considered biologically important regulators of vascular tone.1 Our results show that a stable EET urea agonist/sEH inhibitor constricts rat PA rings in a dose- and calcium-dependent manner, as does 8,9-EET. On the other hand, MAs exhibit no vasoactive response to most eicosanoids. These observations raise new therapeutic opportunities for analogs with the potential targeting of SMCs from a particular vascular bed.
It is interesting to consider the reasons underlying a differential response of PA and MA rings to 8-HUDE. EETs increase intracellular calcium concentrations in aortic vascular SMCs, and all EETs (5,6-EET, 8,9-EET, 11,12-EET, 14,15-EET) are reported to produce similar results.11 In the systemic circulation, EETs have been identified as endothelial-dependent vasodilators.23 In contrast, EETs are proconstrictive in the pulmonary vascular bed.10 Our results demonstrate that both native 8,9-EET and 8-HUDE increase intracellular calcium in rat PASMCs. This activity is attributable to EET-like effect (rather than SEH inhibition) as AUDA alone had no effect on intracellular Ca2+ and it had no influence on the responses to 8-HUDE. It is tempting to speculate that different vasoactive responses may be correlated with differential tissue distribution or regulation of TRPCs, EET receptors or regulation of intracellular store release by EETs due to some other mechanism, but these hypotheses have yet to be tested.
The observation that EETs increase [Ca2+]i in vascular PASMCs is consistent with previous studies in other cell types, although both a potential primary role of EETs in stimulated intracellular calcium release and studies of calcium in PASMCs in response to EETs are new. [Ca2+]i is reported to increase through enhanced influx in ventricular myocytes treated with 14,15-EET,11 5,6-EET or 11,12-EET.24 Graber et al.25 noted that 8,9- and 11,12-EET restored intracellular Ca2+ pools and growth responses in thapsigargin-treated SMC lines. We observed that both native 8,9-EET and 8-HUDE increase intracellular calcium in a manner that depends on both release from intracellular stores (as it occurs in the setting of chelation of extracellular calcium, and is blocked by caffeine, 2-APB and CPA) and calcium influx, as it is blocked by chelation of external calcium or blocking of TRPCs with SKF96365.
Ca2+ influx-induced release of Ca2+ from intracellular stores has an important role in vasoconstriction in many vascular beds. Previous research26, 27 has demonstrated that Ca2+ is released from Ca2+ stores by engagement of IP3 receptors, whereas other studies implicate the ryanodine receptor.28, 29, 30, 31 Our data show that 8-HUDE-induced elevation of Ca2+ in PASMCs from intracellular Ca2+ stores is attributable to activating both IP3 and ryanodine receptors. In fact, activation of ryanodine receptors might be the main mechanism of intracellular Ca2+ release by 8-HUDE, because analog-dependent increases in [Ca2+]i were nearly completely blocked after treatment with caffeine. 2-APB was far less effective in inhibiting 8-HUDE-induced increases in [Ca2+]i. These results hint that the analog may first activate the ryanodine receptor, and then causes activation of IP3 receptors, a scenario that has been suggested to account for Ca2+-induced Ca2+ release.32
Interestingly, pulmonary vascular ring tension was very minimally increased, whereas [Ca2+]i FI in PASMCs was modestly incremented by 8-HUDE in Ca2+-chelated external solutions (Figures 2 and 3). It is possible that because of the limited quantity of Ca2+ in the endoplasmic reticulum, the release of intracellular Ca2+ induced by 8-HUDE may be transient. We speculate that 8-HUDE-induced Ca2+ release may be sufficient to trigger TRPC activation, which then leads to extracellular Ca2+ influx to cause the contraction of PASMCs and to replenish intracellular calcium stores. However, we cannot definitively draw this conclusion because of the fact that caffeine has been reported to inhibit IP3 and other channels33 and our data cannot distinguish between primary effects of 8-HUDE on calcium influx or release from intracellular stores. To our knowledge, there are no reports of direct activation of release of calcium from intracellular stores by EETs. Therefore, our data generally support 8-HUDE-evoked CCE in PASMCs. The specific/independent roles of IP3, sarcoplasmic reticulum or ryanodine receptors, or possible effects of 8-HUDE on TRPC independent of those on intracellular calcium stores in this process remain to be established.
Another finding from this study is that 8-HUDE increases the expression of TRPC and inhibits sEH expression in pulmonary vascular SMCs. The physiological regulation of TRPC expression by endogenous lipids has precedent in the literature.34 For example, EETs activate peroxisome proliferation-activated receptors in HepG2 cells.35 Enhanced TRPC6 expression and increased intracellular calcium are observed in MA SMCs from hypertensive rats.36 Keseru et al.37 linked cytochrome P450-derived eicosatrienoic acids and enhanced TRPC6 expression to hypoxic pulmonary hypertension in rodents.
It is reported that 8-HUDE behaves as both a good EET agonist in coronary artery SMCs and a powerful sEH inhibitor against human recombinant sEH, respectively.7 Intracellular levels of EETs are tightly regulated, and metabolism by sEH, which is the most important EET-metabolizing enzyme, occurs relatively quickly. Although AUDA is a recognized inhibitor of sEH activity, we found that it also decreases the expression of sEH in PASMCs (Figure 8). Inhibition of sEH would increase intracellular EET levels. Increased EETs, regardless of the mechanism by which this is accomplished, has been linked to anti-inflammatory properties and nitric oxide- and prostacyclin-independent vasodilatation in the systemic circulation, but vasoconstriction in the pulmonary circulation.37, 38 The role of naturally occurring EETs or the potential of our EET analog to moderate pulmonary vascular remodeling remains to be evaluated.
In conclusion, 8-HUDE constricts PA rings but not MA rings through coordinated activation of multiple Ca2+ pathways including: (1) activation of the IP3 receptor and/or the caffeine-sensitive ryanodine receptor and then release of Ca2+ stores, likely mainly from the sarcoplasmic reticulum; (2) influx of calcium from extracellular sources through TRPC; (3) upregulation of the expression of TRPCs at both mRNA and protein levels after subacute exposure, which would be expected to lead to elevated CCE by store-operated Ca2+ channels in PASMCs; and (4) decreased expression of sEH. These observations support the potential to develop eicosanoid analogs with targeted and relatively sustained effects on the bioactivity of SMCs from a targeted circulation.
Accession codes
References
Harder DR, Campbell WB, Roman RJ . Role of cytochrome P-450 enzymes and metabolites of arachidonic acid in the control of vascular tone. J Vasc Res 1995; 32: 79–92.
Ellis EF, Police RJ, Yancey L, McKinney JS, Amruthesh SC . Dilation of cerebral arterioles by cytochrome P-450 metabolites of arachidonic acid. Am J Physiol 1990; 259: H1171–H1177.
Katoh T, Takahashi K, Capdevila J, Karara A, Falck JR, Jacobson HR, Badr KF . Glomerular stereospecific synthesis and hemodynamic actions of 8,9-epoxyeicosatrienoic acid in rat kidney. Am J Physiol 1991; 261: F578–F586.
Oyekan AO, McGiff JC, Rosencrantz-Weiss P, Quilley J . Relaxant responses of rabbit aorta: influence of cytochrome P450 inhibitors. J Pharmacol Exp Ther 1994; 268: 262–269.
Campbell WB, Gebremedhin D, Pratt PF, Harder DR . Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 1996; 78: 415–423.
Falck JR, Reddy LM, Reddy YK, Bondlela M, Krishna UM, Ji Y, Sun J, Liao JK . 11,12-epoxyeicosatrienoic acid (11,12-EET): structural determinants for inhibition of TNF-alpha-induced VCAM-1 expression. Bioorg Med Chem Lett 2003; 13: 4011–4014.
Falck JR, Kodela R, Manne R, Atcha KR, Puli N, Dubasi N, Manthati VL, Capdevila JH, Yi XY, Goldman DH, Morisseau C, Hammock BD, Campbell WB . 14,15-Epoxyeicosa-5,8,11-trienoic acid (14,15-EET) surrogates containing epoxide bioisosteres: influence upon vascular relaxation and soluble epoxide hydrolase inhibition. J Med Chem 2009; 52: 5069–5075.
Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R . Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 1999; 401: 493–497.
Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK . Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 1999; 285: 1276–1279.
Zhu D, Bousamra II M, Zeldin DC, Falck JR, Townsley M, Harder DR, Roman RJ, Jacobs ER . Epoxyeicosatrienoic acids constrict isolated pressurized rabbit pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 2000; 278: L335–L343.
Fang X, Weintraub NL, Stoll LL, Spector AA . Epoxyeicosatrienoic acids increase intracellular calcium concentration in vascular smooth muscle cells. Hypertension 1999; 34: 1242–1246.
Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, Hammock BD . Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat Med 1997; 3: 562–566.
Spector AA, Fang X, Snyder GD, Weintraub NL . Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res 2004; 43: 55–90.
Zeldin DC . Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem 2001; 276: 36059–36062.
Xiao YF, Huang L, Morgan JP . Cytochrome P450: a novel system modulating Ca2+ channels and contraction in mammalian heart cells. J Physiol 1998; 508: 777–792.
Guo L, Tang X, Tian H, Liu Y, Wang Z, Wu H, Wang J, Guo S, Zhu D . Subacute hypoxia suppresses Kv3.4 channel expression and whole-cell K+ currents through endogenous 15-hydroxyeicosatetraenoic acid in pulmonary arterial smooth muscle cells. Eur J Pharmacol 2008; 587: 187–195.
Zhu D, Medhora M, Campbell WB, Spitzbarth N, Baker JE, Jacobs ER . Chronic hypoxia activates lung 15-lipoxygenase, which catalyzes production of 15-HETE and enhances constriction in neonatal rabbit pulmonary arteries. Circ Res 2003; 92: 992–1000.
Zheng X, Li Q, Tang X, Liang S, Chen L, Zhang S, Wang Z, Guo L, Zhang R, Zhu D . Source of the elevation Ca2+ evoked by 15-HETE in pulmonary arterial myocytes. Eur J Pharmacol 2008; 601: 16–22.
Ma J, Liang S, Wang Z, Zhang L, Jiang J, Zheng J, Yu L, Zheng X, Wang R, Zhu D . ROCK pathway participates in the processes that 15-hydroxyeicosate-traenoic acid (15-HETE) mediated the pulmonary vascular remodeling induced by hypoxia in rat. J Cell Physiol 2010; 222: 82–94.
Janiak R, Wilson SM, Montague S, Hume JR . Heterogeneity of calcium stores and elementary release events in canine pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 2001; 280: C22–C33.
Peppiatt CM, Collins TJ, Mackenzie L, Conway SJ, Holmes AB, Bootman MD, Berridge MJ, Seo JT, Roderick HL . 2-Aminoethoxydiphenyl borate (2-APB) antagonises inositol 1,4,5-trisphosphate-induced calcium release, inhibits calcium pumps and has a use-dependent and slowly reversible action on store-operated calcium entry channels. Cell Calcium 2003; 34: 97–108.
Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS . Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 2004; 95: 496–505.
Michaelis UR, Fisslthaler B, Barbosa-Sicard E, Falck JR, Fleming I, Busse R . Cytochrome P450 epoxygenases 2C8 and 2C9 are implicated in hypoxia-induced endothelial cell migration and angiogenesis. J Cell Sci 2005; 118: 5489–5498.
Moffat MP, Ward CA, Bend JR, Mock T, Farhangkhoee P, Karmazyn M . Effects of epoxyeicosatrienoic acids on isolated hearts and ventricular myocytes. Am J Physiol 1993; 264: H1154–H1160.
Graber MN, Alfonso A, Gill DL . Recovery of Ca2+ pools and growth in Ca2+ pool-depleted cells is mediated by specific epoxyeicosatrienoic acids derived from arachidonic acid. J Biol Chem 1997; 272: 29546–29553.
Kaplin AI, Snyder SH, Linden DJ . Reduced nicotinamide adenine dinucleotide-selective stimulation of inositol 1,4,5-trisphosphate receptors mediates hypoxic mobilization of calcium. J Neurosci 1996; 16: 2002–2011.
Berridge MJ . Inositol trisphosphate and calcium signalling. Nature 1993; 361: 315–325.
Du W, Frazier M, McMahon TJ, Eu JP . Redox activation of intracellular calcium release channels (ryanodine receptors) in the sustained phase of hypoxia-induced pulmonary vasoconstriction. Chest 2005; 128: 556S–558S.
Jabr RI, Toland H, Gelband CH, Wang XX, Hume JR . Prominent role of intracellular Ca2+ release in hypoxic vasoconstriction of canine pulmonary artery. Br J Pharmacol 1997; 122: 21–30.
Morio Y, McMurtry IF . Ca(2+) release from ryanodine-sensitive store contributes to mechanism of hypoxic vasoconstriction in rat lungs. J Appl Physiol 2002; 92: 527–534.
Zheng YM, Wang QS, Rathore R, Zhang WH, Mazurkiewicz JE, Sorrentino V, Singer HA, Kotlikoff MI, Wang YX . Type-3 ryanodine receptors mediate hypoxia-, but not neurotransmitter-induced calcium release and contraction in pulmonary artery smooth muscle cells. J Gen Physiol 2005; 125: 427–440.
Fabiato A . Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol 1985; 85: 247–289.
Bezprozvanny I, Bezprozvannaya S, Ehrlich BE . Caffeine-induced inhibition of inositol(1,4,5)-trisphosphate-gated calcium channels from cerebellum. Mol Biol Cell 1994; 5: 97–103.
Ramsey IS, Delling M, Clapham DE . An introduction to TRP channels. Annu Rev Physiol 2006; 68: 619–647.
Ng VY, Huang Y, Reddy LM, Falck JR, Lin ET, Kroetz DL . Cytochrome P450 eicosanoids are activators of peroxisome proliferator-activated receptor alpha. Drug Metab Dispos 2007; 35: 1126–1134.
Zulian A, Baryshnikov SG, Linde CI, Hamlyn JM, Ferrari P, Golovina VA . Upregulation of Na+/Ca2+ exchanger and TRPC6 contributes to abnormal Ca2+ homeostasis in arterial smooth muscle cells from Milan hypertensive rats. Am J Physiol Heart Circ Physiol 2010; 299: H624–H633.
Keseru B, Barbosa-Sicard E, Popp R, Fisslthaler B, Dietrich A, Gudermann T, Hammock BD, Falck JR, Weissmann N, Busse R, Fleming I . Epoxyeicosatrienoic acids and the soluble epoxide hydrolase are determinants of pulmonary artery pressure and the acute hypoxic pulmonary vasoconstrictor response. FASEB J 2008; 22: 4306–4315.
Michaelis UR, Fleming I . From endothelium-derived hyperpolarizing factor (EDHF) to angiogenesis: epoxyeicosatrienoic acids (EETs) and cell signaling. Pharmacol Ther 2006; 111: 584–595.
Acknowledgements
This study was supported by the National Natural Science Foundation of China Grant no. 30470752.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, Y., Wang, R., Li, J. et al. Stable EET urea agonist and soluble epoxide hydrolase inhibitor regulate rat pulmonary arteries through TRPCs. Hypertens Res 34, 630–639 (2011). https://doi.org/10.1038/hr.2011.5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2011.5
Keywords
This article is cited by
-
TRPC5 ion channel permeation promotes weight gain in hypercholesterolaemic mice
Scientific Reports (2019)