Abstract
It has been suggested that proangiotensin-12 (proang-12), a novel angiotensin peptide recently discovered in rat tissues, may function as a component of the tissue renin-angiotensin system (RAS). To investigate the role of proang-12 in the production of angiotensin II (Ang II), we measured its plasma and tissue concentrations in Wistar–Kyoto (WKY) and spontaneously hypertensive (SHR) rats, with and without RAS inhibition. The 15-week-old male WKY and SHR rats were left untreated or were treated for 7 days with 30 mg kg−1 per day losartan, an angiotensin receptor blocker, or with 20 mg kg−1 per day imidapril, an angiotensin-converting enzyme (ACE) inhibitor. Both treatments increased renin activity and the concentrations of angiotensin I (Ang I) and Ang II in the plasma of WKY and SHR rats, but neither affected plasma proang-12 levels. In contrast to the comparatively low level of proang-12 seen in plasma, cardiac and renal levels of proang-12 were higher than those of Ang I and Ang II. In addition, despite activation of the RAS in the systemic circulation, tissue concentrations of proang-12 were significantly reduced following treatment with losartan or imidapril. Similar reductions were also observed in the tissue concentrations of Ang II in both strains, without a reduction in Ang I. These results suggest that tissue concentrations of proang-12 and Ang II are regulated independently of the systemic RAS in WKY and SHR rats, which is consistent with the notion that proang-12 is a component of only the tissue RAS.
Similar content being viewed by others
Introduction
The renin-angiotensin system (RAS) has a crucial role in the regulation of blood pressure and fluid balance. It is well established that renin secreted from the kidneys cleaves angiotensinogen circulating in the blood to produce angiotensin I (Ang I), which is in turn cleaved by angiotensin-converting enzyme (ACE) to produce angiotensin II (Ang II), a potent pressor peptide mediating the major actions of the circulating (systemic) RAS.1, 2, 3 By contrast, much less is known about the tissue RAS, and many questions about the angiotensin processing cascade and the role of the tissue RAS in regulating blood pressure and fluid balance remain unanswered.4, 5, 6, 7
Proangiotensin-12 (proang-12) is a 12-amino acid, C-terminal extended form of Ang I, which we recently isolated from rat small intestine.8 In vitro, proang-12 constricts aortic strips and, when intravenously infused into rats, raises blood pressure. The vasoconstrictor and pressor effects of proang-12 are abolished by ACE inhibitors and angiotensin receptor blockers, which suggests ACE is involved in the conversion of proang-12 to Ang II.8, 9, 10 However, Prosser et al.11, 12 reported that chymase inhibition attenuates proang-12-induced cardiac damage caused by ischemia-reperfusion in rat hearts ex vivo, as well as proang-12-induced constriction of isolated rat arteries. This suggests that chymase is also involved in the conversion of proang-12 to Ang II. Moreover, Ahmad et al.13 demonstrated that ACE, neprilysin and chymase are all involved in the metabolism of proang-12 in neonatal cardiac myocytes. Taken together, these findings suggest proang-12 may be metabolized or converted to Ang II by several enzymes. In addition, proang-12 is also metabolized to two other angiotensin-related peptides, Ang(1–7)14 or Ang(1–9).15
In various tissues, including the heart and kidneys, the concentration of proang-12 is much higher than those of Ang I and Ang II.8, 9, 16 By contrast, the concentration of proang-12 in plasma is lower than that of either Ang I or Ang II. This suggests that proang-12 may be a component of only the tissue RAS in rats. To investigate the role of systemic renin in the production of proang-12, we previously measured tissue proang-12 concentrations in rats subjected to bilateral nephrectomy17 or fed a low-salt diet.18 Both of these experiments showed that tissue proang-12 is regulated in a manner that is independent of the plasma renin activity.
Therefore, our aim in the present study was to clarify the role of proang-12 in the production of Ang II in tissue and blood. To accomplish this, we assessed the plasma and tissue concentrations of Ang II, Ang I and proang-12 in Wistar–Kyoto (WKY) and spontaneously hypertensive (SHR) rats, with and without RAS inhibitor.
Methods
Animal and RAS inhibitors
The 15-week-old male WKY and SHR rats were purchased from Charles River Laboratories (Kanagawa, Japan). Losartan and imidapril were kindly provided by Merck (Whitehouse Station, NJ, USA) and Mitsubishi Tanabe Pharma Corporation (Osaka, Japan), respectively.
Experimental protocol
The rats were maintained under a 12-h light/12-h dark cycle and specific pathogen-free conditions, and were fed a normal diet. Before experimentation, the rats were randomly divided into three groups (n=6–8 in each group): the control group was left untreated; the losartan group received 30 mg kg−1 losartan in their drinking water daily for 7 days; and the imidapril group received 20 mg kg−1 imidapril daily over the same period. We selected doses of both agents that reportedly suppress the systemic RAS to a substantial degree.19, 20 Blood pressures were measured using the tail-cuff method (model BP-98A; Softron, Tokyo, Japan), before and after treatment. At the end of the treatment period the rats were decapitated, and blood samples were collected into tubes containing 10 mg ml−1 EDTA and 500 KIU ml−1 aprotinin, and were then immediately centrifuged for 10 min (3000 g and 4 °C) to obtain the plasma.
The present study was performed in accordance with the Animal Welfare Act and with the approval of the University of Miyazaki Institutional Animal Care and Use Committee (2008-501-2).
Sample preparation for radioimmunoassay (RIA)
Samples were prepared for RIA as described previously.21, 22 After decapitating the rats, tissues of interest were carefully resected and boiled for 10 min in 10 volumes of distilled H2O. Acetic acid was then added to the samples to a final concentration of 1.0 mol l−1, and the samples were homogenized using a Polytron mixer and then centrifuged for 20 min (12 000 r.p.m. at 4 °C). Finally, the plasma and tissue samples were separately applied to a Sep-Pak C18 cartridge and eluted with 60% acetonitrile in 0.1% trifluoroacetic acid. The eluted samples were lyophilized and stored at −20 °C until used for RIA.
Measurement of proang-12 and other components of the RAS
To specifically detect proang-12 in tissues and plasma, we developed an RIA using antiserum raised against the C-terminal portion of the peptide. The details of this RIA, including its cross-reactivity with other angiotensin peptides and comparison with a high-performance liquid chromatographic analysis of immunoreactive proang-12, are provided elsewhere.8 The Ang I concentrations in tissues and plasma were determined using a specific RIA developed using an antibody raised against the C-terminus of Ang I (Miles). Ang II concentrations were measured using a RIA developed against Ang II antiserum (Cortex Biochem., San Leandro, USA).8 The Ang I RIA cross-reacted with proang-12 at a level of 1.6%; there was no cross-reaction with Ang II. The Ang II RIA showed no cross-reactivity with either proang-12 or Ang I. The angiotensinogen concentrations in plasma were determined using an ELISA purchased from Immuno-Biological Laboratories (Gunma, Japan).21, 22 Plasma renin activity was determined using a GammaCoat Plasma Renin Activity kit from Kyowa Medex (Tokyo, Japan). Estimates of renin activity were based on the plasma concentrations of generated Ang I following incubation at 37 °C.
Statistical analysis
Data are presented as means±s.e. Comparisons of data from multiple groups were made using ANOVA followed by the Tukey–Kramer test. Values of P<0.05 were considered significant.
Results
Table 1 shows the systolic and diastolic blood pressures and heart/body weight ratios of WKY and SHR rats, before and after treatment. Both systolic blood pressure and diastolic blood pressure were significantly lower than control in imidapril-treated WKY rats, but not in WKY rats treated with losartan. Heart/body weight ratios in WKY rats were unaffected by either losartan or imidapril. In SHR rats, both losartan and imidapril significantly reduced systolic blood pressure and diastolic blood pressure, as compared with control, and the heart/body weight ratios were slightly lower than control in the losartan group and significantly lower in the imidapril group.
Figure 1 shows the renin activity and angiotensinogen concentrations in plasma from WKY and SHR rats left untreated or treated with a RAS inhibitor. We found that plasma renin activity was significantly higher than control in the losartan and imidapril groups of both strains (Figures 1a and b). The plasma angiotensinogen concentration remained unchanged in the three groups of WKY rats (Figure 1c), but was significantly higher in losartan-treated SHR rats than in the untreated controls (Figure 1d).
Plasma concentrations of proang-12, Ang I and Ang II in WKY and SHR rats are shown in Figure 2. RAS inhibition had no significant effect on plasma proang-12 concentrations in either WKY or SHR rats (Figures 2a and b). On the other hand, plasma Ang I concentrations were significantly higher than control in both strains treated with losartan or imidapril (Figures 2c and d). Although the effect was not statistically significant, we also observed substantial elevations in plasma Ang II concentrations in WKY rats treated with losartan or imidapril (Figure 2e), and a significant elevation in plasma Ang II was seen in SHR rats treated with imidapril (Figure 2f).
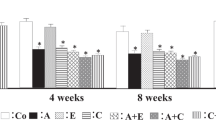
We also measured the tissue concentrations of proang-12, Ang I and Ang II in the cardiac left ventricle and kidneys, two regions in which the tissue RAS is thought to be active (Figures 3 and 4). We noted a consistent tendency for both losartan and imidapril to reduce left ventricular and renal levels of proang-12 and Ang II in both the WKY and SHR rats, but the effect was not always statistically significant (Figures 3a, b, e, f and 4a, b, e and f). By contrast, losartan and imidapril had little, if any, effect on Ang I levels. The one exception was a small but significant rise in renal Ang I levels in WKY rats (Figures 3c, d and 4c and d).
Discussion
In the present study, plasma proang-12 concentrations were unaffected by pharmacological inhibition of the systemic RAS for 7 days using losartan or imidapril; this was despite elevations in plasma renin activity and Ang I and Ang II levels. These results are consistent with our earlier finding that plasma proang-12 levels in rats were unaffected by restricting sodium intake, though systemic RAS activity, including plasma renin activity, was significantly activated.18 Apparently, renin is not involved in the synthesis of proang-12, which is consistent renin functioning exclusively to cleave angiotensinogen to produce Ang I.1, 23 It should be noted that the plasma concentrations of proang-12 are much lower than those of Ang II and Ang I. Particularly in animals treated with imidapril, the plasma proang-12 concentration was less than 1% of the Ang I concentration, suggesting endogenous proang-12 does not contribute the production of Ang II in plasma.
In the present study, left ventricular and renal proang-12 concentrations were higher than the Ang II and Ang I concentrations in both SHR and WKY rats. Moreover, the concentrations of proang-12 and Ang II in both heart and kidney showed similar changes in rats treated with an ACE inhibitor. On the other hand, the tissue levels of Ang II did not correlate well with those of Ang I, which suggests tissue Ang II production is affected by proang-12, but not by Ang I.
It was unexpected that imidapril would reduce tissue levels of proang-12 and Ang II in both WKY and SHR rats under conditions in which ACE was inhibited. We cannot be certain of the exact mechanism underlying the suppression of local proang-12 production by the ACE inhibitor, but two possible explanations come to mind. One possibility is that ACE inhibitors or angiotensin receptor blockers reduce the expression of angiotensinogen in cardiac and renal tissue. Wagner et al.24 reported that plasma angiotensinogen is reduced in the stroke prone strain of SHR rats when the animals are treated with captopril or losartan. Another possibility is that metabolism of proang-12 is catalyzed by several proteases. For example, proang-12 was recently shown to be digested by ACE2, chymase and neprilysin, in addition to ACE. What's more, proang-12 is reportedly converted to Ang I, Ang II, Ang -(1–9) and Ang-(1–7) ex vivo,14, 15 and losartan, olmesartan and lisinopril all increase rat cardiac ACE2 expression, leading to increases in Ang-(1–7) levels25, 26 and chymase activation.27 Thus, ACE inhibitors and angiotensin receptor blockers appear to activate endogenous proteases that metabolize proang-12.
It is somewhat surprising that imidapril treatment increased plasma Ang II levels, despite the reduction of blood pressure. Similarly, plasma Ang II concentrations were previously reported to be significantly increased by enalapril28, 29 and imidapril,30 despite reductions in systolic blood pressure, left ventricular weight and left ventricular Ang II concentration.31 The mechanism underlying these findings remains unclear, but one possible explanation is the presence of an ACE-independent pathway for Ang II production, which may be activated during pharmacological ACE inhibition; for example, chymase is reportedly activated by captopril treatment.27 Another mechanism that could be involved in the imidapril-induced reduction in blood pressure is ACE inhibitor-mediated activation of ACE2, leading to increases in Ang (1–7) levels.25 In addition, metabolism of bradykinin is strongly inhibited by ACE inhibitors, and the resultant increases circulating bradykinin levels would lead to increases in nitric oxide and prostaglandin levels,32 which would in turn reduce blood pressure.
We also observed that tissue proang-12 levels were higher in WKY than SHR rats. This finding differs from that of an earlier study, which found that cardiac levels of proang-12 were higher in SHR than WKY rats, but that the reverse was true for renal proang-12 levels.16 We are uncertain what accounts for the difference between our findings and those earlier ones. Perhaps it reflects the fact that the rats were bred in different colonies or that the extraction and assay procedures differed. In any case, the angiotensin peptides showed similar changes in both strains, suggesting proang-12 is an important precursor of tissue Ang II.
In future experiments, attention should be paid to the enzymes involved in the processing or conversion of proang-12, and the role of proang-12 in regulating blood pressure and fluid balance. This is because those enzymes may be key regulators of the local production of both Ang II and proang-12 from angiotensinogen, which would make them key regulators of the tissue RAS. In addition, an understanding of the handling of renal/urinary angiotensinogen is necessary to further clarify the biosynthesis of proang-12 in tissues.
Conclusion
The left ventricular and renal concentrations of proang-12 are reduced by losartan and imidapril in WKY and SHR rats, despite activation of the circulation RAS. Whereas the circulation RAS involves a pathway via which Ang I is converted to Ang II, the tissue RAS involves conversion of proang-12 to Ang II. It is therefore likely that tissue concentrations of proang-12 are regulated independently of the circulation RAS, and that proang-12 is a component of only the tissue RAS in rats.
References
Oparil S, Haber E . The renin-angiotensin system (first of two parts). N Engl J Med 1974; 291: 389–401.
Dzau VJ . Significance of the vascular renin-angiotensin pathway. Hypertension 1986; 8: 553–559.
Dzau VJ, Burt DW, Pratt RE . Molecular biology of the renin-angiotensin system. Am J Physiol 1988; 255 (4 Pt 2): F563–F573.
Campbell DJ . Circulating and tissue angiotensin systems. J Clin Invest 1987; 79: 1–6.
Fischer-Ferraro C, Nahmod VE, Goldstein DJ, Finkielman S . Angiotensin and renin in rat and dog brain. J Exp Med 1971; 133: 353–361.
Miyazaki M, Okunishi H, Okamura T, Toda N . Elevated vascular angiotensin converting enzyme in chronic two-kidney, one clip hypertension in the dog. J Hypertens 1987; 5: 155–160.
Paul M, Poyan Mehr A, Kreutz R . Physiology of local renin-angiotensin systems. Physiol Rev 2006; 86: 747–803.
Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K . Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun 2006; 350: 1026–1031.
Varagic J, Trask AJ, Jessup JA, Chappell MC, Ferrario CM . New angiotensins. J Mol Med 2008; 86: 663–671.
Cummins PM . A new addition to the renin-angiotensin peptide family: proAngiotensin-12 (PA12). Cardiovasc Res 2009; 82: 7–8.
Prosser HC, Forster ME, Richards AM, Pemberton CJ . Cardiac chymase converts rat proAngiotensin-12 (PA12) to angiotensin II: effects of PA12 upon cardiac haemodynamics. Cardiovasc Res 2009; 82: 40–50.
Prosser HC, Richards AM, Forster ME, Pemberton CJ . Regional vascular response to ProAngiotensin-12 (PA12) through the rat arterial system. Peptides 2010; 31: 1540–1545.
Ahmad S, Varagic J, Westwood BM, Chappell MC, Ferrario CM . Uptake and metabolism of the novel Peptide Angiotensin-(1-12) by neonatal cardiac myocytes. PLoS ONE 2011; 6: e15759.
Trask AJ, Jessup JA, Chappell MC, Ferrario CM . Angiotensin-(1-12) is an alternate substrate for angiotensin peptide production in the heart. Am J Physiol Heart Circ Physiol 2008; 294: H2242–H2247.
Bujak-Gizycka B, Olszanecki R, Suski M, Madek J, Stachowicz A, Korbut R . Angiotensinogen metabolism in rat aorta: robust formation of proangiotensin-12. J Physiol Pharmacol 2010; 61: 679–682.
Jessup JA, Trask AJ, Chappell MC, Nagata S, Kato J, Kitamura K, Ferrario CM . Localization of the novel angiotensin peptide, angiotensin-(1-12), in heart and kidney of hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol 2008; 294: H2614–H2618.
Ferrario CM, Varagic J, Habibi J, Nagata S, Kato J, Chappell MC, Trask AJ, Kitamura K, Whaley-Connell A, Sowers JR . Differential regulation of angiotensin-(1-12) in plasma and cardiac tissue in response to bilateral nephrectomy. Am J Physiol Heart Circ Physiol 2009; 296: H1184–H1192.
Nagata S, Kato J, Kuwasako K, Kitamura K . Plasma and tissue levels of proangiotensin-12 and components of the renin-angiotensin system (RAS) following low- or high-salt feeding in rats. Peptides 2010; 31: 889–892.
Dai Q, Xu M, Yao M, Sun B . Angiotensin AT1 receptor antagonists exert anti-inflammatory effects in spontaneously hypertensive rats. Br J Pharmacol 2007; 152: 1042–1048.
Katoh M, Egashira K, Kataoka C, Usui M, Koyanagi M, Kitamoto S, Ohmachi Y, Takeshita A, Narita H . Regression by ACE inhibition of arteriosclerotic changes induced by chronic blockade of NO synthesis in rats. Am J Physiol Heart Circ Physiol 2001; 280: H2306–H2312.
Kobori H, Katsurada A, Miyata K, Ohashi N, Satou R, Saito T, Hagiwara Y, Miyashita K, Navar LG . Determination of plasma and urinary angiotensinogen levels in rodents by newly developed ELISA. Am J Physiol Renal Physiol 2008; 294: F1257–F1263.
Katsurada A, Hagiwara Y, Miyashita K, Satou R, Miyata K, Ohashi N, Navar LG, Kobori H . Novel sandwich ELISA for human angiotensinogen. Am J Physiol Renal Physiol 2007; 293: F956–F960.
Inagami T, Misono K, Michelakis AM . Definitive evidence for similarity in the active site of renin and acidic protease. Biochem Biophys Res Commun 1974; 56: 503–509.
Wagner J, Drab M, Bohlender J, Amann K, Wienen W, Ganten D . Effects of AT1 receptor blockade on blood pressure and the renin-angiotensin system in spontaneously hypertensive rats of the stroke prone strain. Clin Exp Hypertens 1998; 20: 205–221.
Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE . Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005; 111: 2605–2610.
Agata J, Ura N, Yoshida H, Shinshi Y, Sasaki H, Hyakkoku M, Taniguchi S, Shimamoto K . Olmesartan is an angiotensin II receptor blocker with an inhibitory effect on angiotensin-converting enzyme. Hypertens Res 2006; 29: 865–874.
Wei CC, Hase N, Inoue Y, Bradley EW, Yahiro E, Li M, Naqvi N, Powell PC, Shi K, Takahashi Y, Saku K, Urata H, Dell′italia LJ, Husain A . Mast cell chymase limits the cardiac efficacy of Ang I-converting enzyme inhibitor therapy in rodents. J Clin Invest 2010; 120: 1229–1239.
Mento PF, Wilkes BM . Plasma angiotensins and blood pressure during converting enzyme inhibition. Hypertension 1987; 9 (6 Pt 2): III42–III48.
Koji T, Onishi K, Dohi K, Okamoto R, Tanabe M, Kitamura T, Ito M, Isaka N, Nobori T, Nakano T . Addition of angiotensin II receptor antagonist to an ACE inhibitor in heart failure improves cardiovascular function by a bradykinin-mediated mechanism. J Cardiovasc Pharmacol 2003; 41: 632–639.
Matsumoto N, Ishimitsu T, Okamura A, Seta H, Takahashi M, Matsuoka H . Effects of imidapril on left ventricular mass in chronic hemodialysis patients. Hypertens Res 2006; 29: 253–260.
Nagano M, Higaki J, Mikami H, Nakamaru M, Higashimori K, Katahira K, Tabuchi Y, Moriguchi A, Nakamura F, Ogihara T . Converting enzyme inhibitors regressed cardiac hypertrophy and reduced tissue angiotensin II in spontaneously hypertensive rats. J Hypertens 1991; 9: 595–599.
Aihara E, Kagawa S, Hayashi M, Takeuchi K . ACE inhibitor and AT1 antagonist stimulate duodenal HCO3-secretion mediated by a common pathway—involvement of PG, NO and bradykinin. J Physiol Pharmacol 2005; 56: 391–406.
Acknowledgements
The present study was supported in part by Grants-in-aid for Scientific Research from the Japan Society for the Promotion of Science. We are grateful to Merck and Mitsubishi Tanabe Pharma Corporation for kindly providing the RAS inhibitors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Nagata, S., Kato, J., Kuwasako, K. et al. Plasma and tissue concentrations of proangiotensin-12 in rats treated with inhibitors of the renin-angiotensin system. Hypertens Res 35, 234–238 (2012). https://doi.org/10.1038/hr.2011.165
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2011.165
Keywords
This article is cited by
-
Does the Naked Emperor Parable Apply to Current Perceptions of the Contribution of Renin Angiotensin System Inhibition in Hypertension?
Current Hypertension Reports (2022)
-
Estrogen modulates the differential expression of cardiac myocyte chymase isoforms and diastolic function
Molecular and Cellular Biochemistry (2019)
-
Novel Cardiac Intracrine Mechanisms Based on Ang-(1-12)/Chymase Axis Require a Revision of Therapeutic Approaches in Human Heart Disease
Current Hypertension Reports (2017)
-
Regulation of a novel angiotensin II precursor, proangiotensin-12, in the tissues by blockade of the renin–angiotensin system
Hypertension Research (2012)