Abstract
A systematic literature review was conducted using PubMed, Embase and the Cochrane Library to determine the effect of acute, chronic and passive smoking on arterial stiffness and to determine whether these effects are reversible after smoking cessation. A total of 39 relevant studies were identified and included. Acute smoking was found to cause an acute increase in arterial stiffness. Similarly, passive smoking increased arterial stiffness acutely and chronically. The majority of studies identified chronic smoking as a risk factor for increasing arterial stiffness. However, some studies found no statistical difference in arterial stiffness between nonsmokers and long-term smokers, although chronic smoking seems to sensitize the arterial response to acute smoking. In addition, whether arterial stiffness is reversed after smoking cessation and the timeline in which this may occur could not be determined from the identified literature. The effect of smoking discontinuation on arterial stiffness remains to be established by prospective smoking cessation trials.
Similar content being viewed by others
Introduction
Cigarette smoking has long been established as a cardiovascular risk factor and is the major preventable cause of death and disability in developed and developing countries.1, 2 Smoking accounts for ∼1 200 000 deaths each year in Europe, 440 000 in the United States and 50 000 in Canada.1, 2, 3 Smoking can cause cardiovascular damage by inducing endothelial dysfunction and harmful hemodynamic effects, such as increased arterial stiffness.4, 5, 6 Furthermore, a number of factors and conditions, such as alcohol consumption, dyslipidemia, hypertension, renal dysfunction, obesity and chronic obstructive pulmonary disease cause changes in arterial stiffness.7, 8, 9, 10, 11, 12, 13, 14 These effects may interact with the effects of smoking to alter arterial stiffness and cardiovascular risk profiles.
The measurement of vessel hemodynamics is gaining popularity as a means to assess arterial stiffness and endothelial function. Accordingly, various noninvasive methods have been developed to measure arterial stiffness, including applanation tonometry, echotracking, Doppler and ultrasound.15 These techniques could be useful in clinical practice, particularly applanation tonometry, as numerous studies have shown that increased arterial stiffness as measured by aortic pulse wave velocity (PWV) and augmentation index (AIx) is directly and independently associated with increased risk of cardiovascular complications and events.16, 17, 18, 19, 20, 21, 22 Although aortic PWV (typically measured by carotid-femoral PWV (cfPWV)) has the most evidence to support its predictive value for cardiovascular events, it is important to understand the differences between PWV measured in various vascular beds; smoking may affect particular areas of the vascular system in different ways. cfPWV reflects the stiffness of the central elastic arteries, crPWV (carotid-radial PWV) and brPWV (brachial-radial PWV) reflect the stiffness of peripheral muscular arteries, and brachial-ankle PWV (baPWV) is an intermediate measurement, providing information about the stiffness of both peripheral muscular and central elastic arteries.15, 16
The purpose of this systematic review was to assess the effect of acute, chronic and passive smoking on arterial stiffness, as well as the effect of smoking cessation on arterial stiffness.
Methods
Data sources and study selection
Studies were identified through PubMed, Embase and the Cochrane Library using the keywords shown in Figure 1. Relevant articles were extracted using search terms, reference lists and the ‘related article’ links of articles selected for review. We included all relevant clinical studies that enrolled chronic smokers and nonsmokers in whom arterial stiffness was measured. The search for articles was limited to those published between 1985 and November 2009, and written in English. Two independent researchers (RJD and AH) conducted the search.
Results
A total of 39 studies were identified, confirmed by all authors and included in this review. Figure 1 illustrates the search and study selection procedure. Tables 1,2,3,4 summarize the findings of the included studies, and Supplementary Tables 1–4 summarize the patient characteristics of the included studies.
Acute smoking
The acute effects of cigarette smoking on arterial stiffness have been explored previously (Table 1). A number of studies have shown that arterial stiffness increases acutely after cigarette or cigar smoking as measured by AIx, cfPWV, brPWV or crPWV.23, 24, 25, 26 Another study between chronic and nonsmokers reported that at baseline, the baPWV was not significantly different but was significantly higher in chronic smokers 5 min after cigarette smoking and remained higher for 30 min (P<0.05); in both groups, baPWV was significantly higher at 5 min when compared with baseline (both P<0.001) and returned to baseline at 45 min.27
Rhee et al.28 reported a significant smoking-induced acute increase in heart-femoral PWV (P<0.05). However, after adjustment for total cholesterol, time-dependent heart rate and brachial mean arterial pressure, this association lost statistical significance.
The acute effects of smoking were also evaluated on aortic, carotid, radial and brachial distensibility and compliance; in all studies, distensibility and compliance decreased in all arterial beds studied.29, 30, 31, 32, 33, 34 Swampillai et al.35 reported a significant smoking-induced increase in stiffness index (SI) 15 min after smoking (P<0.05), but did not observe changes in wave speed in the carotid artery.
Chronic smoking
Several studies reported the relationship between PWV and smoking (Table 2). In one study, brPWV was significantly increased in smokers;36 another study reported higher PWV in smokers, but did not report the arterial segment that was measured.37 Similarly, increases in aortic stiffness were also noted in smokers in other studies.38, 39 In fact, cigarette smoking was found to be a significant predictor of increased aortic PWV in White and Black individuals (R2=0.13 and R2=0.14, respectively; P<0.05).39 In contrast, Yufu et al.40 found no significant differences between smokers and nonsmokers when evaluating baPWV. In addition, three different groups found no significant correlation between smoking status and increased cfPWV.41, 42, 43 However, AIx was increased in smokers (17.25%) compared with nonsmokers (11.75%; P=0.004) in one of the same subject populations.42 A number of other studies found AIx to be increased in chronic smokers compared with nonsmokers.24, 41, 44, 45 Furthermore, Tomiyama et al.46 showed that AIx was independently correlated with smoking status in men and women.
Some of the identified studies evaluated arterial stiffness using ultrasonographic methods: SI, distensibility and compliance. Two studies reported that smokers had significant increases in the SI when compared with nonsmokers.47, 48 However, another study only reported significant increases in SI in female smokers (P=0.041).49 Aortic compliance50 and aortic distensibility51 were significantly decreased in smokers compared with nonsmokers. In a study of smoking adolescents, the normalized pressure strain in smokers was higher than in nonsmokers (P=0.001).52 McVeigh et al.53 found that the damping of diastolic oscillation was significantly increased in smokers, and that the derived oscillatory compliance estimate was reduced compared with nonsmokers. Another study also found stiffer arteries in chronic smokers; however, this was limited to the popliteal artery.54 Nevertheless, one study found no difference in carotid and brachial artery distensibility between nonsmokers and chronic smokers.32
Passive smoking
Three different studies found that exposure to environmental tobacco smoke (ETS) for 5–60 min leads to increased arterial stiffness (Table 3).34, 55, 56 However, in one study, this change was noted only in male subjects.56 No change was observed after exposure to nontobacco smoke in any of the studies.34, 55, 56 Interestingly, administration of sublingual nicotine was associated with increased AIx (P=0.001).55 Another study reported a significant correlation between carotid arterial SI and ETS exposure. In older individuals, or in those with increased intima–media thickness, the adjusted SI was increased with increasing number of ETS sources (P=0.09 and P=0.05, respectively). In individuals with a higher body mass index, or in those with increased intima–media thickness, the adjusted SI was increased within hours of exposure to ETS (both P=0.04).57
Smoking cessation
The effect of smoking cessation in reversing increased arterial stiffness is not well established (Table 4). Oren et al.58 and Polonia et al.59 observed a decrease in AIx after 6 months of smoking cessation; continuing smokers showed no such change.58, 59 Rehill et al.42 reported similar results after only 4 weeks of smoking cessation. In contrast, another study reported no significant change in arterial stiffness between persistent smokers and in individuals who stopped smoking for 2 years as assessed by the distensibility of the carotid and femoral arteries.60 Furthermore, Polonia et al.59 found that no change occurred in cfPWV after 6 months of smoking cessation.
Discussion
In this systematic review, we presented results obtained from 39 studies evaluating the effect of smoking on arterial stiffness. These studies show that acute, chronic and passive smoking has a detrimental effect on arterial stiffness, and that smoking cessation may improve arterial stiffness. However, further work is required to definitively determine whether arterial stiffness is improved over time after smoking cessation and the timeline in which this occurs.
The effect of acute smoking is clear cut, causing an acute increase in arterial stiffness in both chronic smokers and nonsmokers. All studies, except one, found that acute smoking causes a significant increase in arterial stiffness in chronic smokers. The study that found no effect was small (30 patients) which actually showed, before adjustment, an increase in heart-femoral PWV after acute smoking.28 Interestingly, chronic smokers had a greater increase in arterial stiffness after acute smoking than did nonsmokers.24, 27 These results are in line with those of previous studies measuring acute smoke-induced endothelial dysfunction measured by flow-mediated dilatation (FMD); endothelial dysfunction was more prolonged in smokers than in nonsmokers.61
The effect of chronic smoking on endothelial dysfunction is also detrimental; studies comparing the FMD of habitual smokers and nonsmokers found smoking to be associated with greater endothelial dysfunction.6, 62 However, the effect of chronic smoking on arterial stiffness is slightly more controversial. Most studies found that chronic smoking increases arterial stiffness as measured by PWV, AIx, SI, compliance and distensibility.24, 36, 37, 38, 39, 47, 48, 49, 50, 51, 52, 53, 63 However, a few other studies did not consistently find this effect.32, 40, 41, 42 For example, Filipovsky et al.41 and Rehill et al.42 found that AIx was significantly higher in smokers than in nonsmokers, while differences in PWV did not reach significance. Kool et al.32 also did not find significant differences between smokers and nonsmokers when measuring compliance and distensibility; however, they only included 14 nonsmokers. Yufu et al. also failed to show a significant difference in baPWV between smokers and nonsmokers; however, they included young, healthy smokers with small cumulative tobacco consumption. Furthermore, in this study, the FMD was strongly correlated with the baPWV (P<0.005).40 It is also noteworthy that PWV was not measured in the same sites in the vascular bed between studies.
Passive smoking clearly causes increased arterial stiffness. Three studies showed that acute exposure to ETS leads to increased stiffness,34, 55, 56 whereas one study found an association between the increased number of ETS sources and the increased time of exposure with a higher SI.57 Furthermore, sublingual nicotine administration was shown to increase AIx, indicating that the effect of smoking on arterial stiffness may act through nicotine.55 Second-hand smoke also causes endothelial dysfunction;5, 64 a study comparing the endothelial function of nonsmokers who were currently or previously exposed to second-hand smoke found that being exposed for ⩽1 h per day for ⩽2 years can cause endothelial dysfunction lasting as long as 2 years after cessation of exposure.64
It has been suggested that smoking-induced damage to the endothelium may be somewhat reversible; former active and passive smokers have better endothelial function than do current active or passive smokers.65, 66 The beneficial effects of smoking cessation on the risk for cardiovascular disease67, 68, 69 and for endothelial dysfunction65, 66 have been established. However, it remains controversial whether arterial stiffness improves after smoking cessation; some studies show rapid improvement in AIx after smoking cessation,42, 58, 59 whereas cfPWV did not improve after 6 months,59 and another study showed no improvement in distensibility after 2 years.59, 60 A cross-sectional study of middle-aged hypertensive patients found that AIx, cfPWV and Tr (transit time) returned to baseline levels only after 10 years of smoking cessation.70 These inconclusive results emphasize the need for long-term longitudinal studies to clarify the controversy on the effect of smoking cessation on arterial stiffness.
Mechanisms
Lipids
Active and passive smoking alters lipid metabolism acutely and chronically (Figure 2).71, 72 Cigarette smoke alters catecholamine release and lipoprotein lipase activity; consequently, free fatty acid release is altered and triglycerides cannot be cleared from the blood.72 This causes an increase in triglycerides and low-density lipoprotein, and a decrease in high-density lipoprotein in the plasma of smokers.71, 72 Changes in lipid metabolism contribute to structural changes of the arterial wall including intima–media thickening and atherogenesis73, 74 and may lead to an increase in arterial stiffness. Furthermore, treatment of the underlying hypercholesterolemia using statins has been shown to decrease arterial stiffness.75, 76
Kidney function
Chronic smoking damages the kidney by causing a loss of filtration rate and contributes to kidney failure.77, 78 In fact, smoking is also correlated with albuminuria in healthy individuals.78 As even mild renal insufficiency leads to collagen accumulation in elastic arteries as well as calcification,79 these smoking-induced alterations in kidney function increase arterial stiffness by altering the structure of the arteries.
Insulin resistance
Smoking increases insulin release and causes insulin resistance,72, 80 both of which have been shown to increase arterial stiffness.81, 82, 83 Gordin et al.81 showed that mean daily glucose correlated with aortic PWV in diabetic subjects. Similar results have been found in various patient groups.82, 83 In fact, aortic PWV has been shown to increase even in early insulin-resistance states.83 Smoking is also frequently associated with decreased physical activity and poor diet, further increasing adiposity and insulin-resistant states.80
Oxidative stress
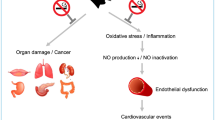
Acute and chronic smoking has been shown to induce oxidative stress, altering vascular tone and increasing arterial stiffness.4, 84 Smoking increases the production of reactive oxygen species, which decreases the activity of nitric oxide synthase (NOS), inhibiting nitric oxide (NO) production by the endothelium.85, 86 Platelet-derived NO production also decreases contributing to a hypercoagulable state.87 Moreover, active and passive smoking decrease total body antioxidant levels,71 which increases endothelial dysfunction and arterial stiffness.85, 88, 89 In fact, total antioxidant capacity is inversely associated with AIx (r=−0.24, P<0.02).82 Treatment of smokers with antioxidants or L-arginine, a NOS substrate, improves FMD and arterial stiffness.85, 90
Inflammation
Smoking has also been shown to cause an inflammatory state by simultaneously increasing proinflammatory and decreasing anti-inflammatory cytokines.91 Various studies have shown strong associations between inflammatory markers and arterial stiffness.92, 93, 94 For example, Vlachopoulos et al. used a Salmonella typhi vaccine to induce inflammation in healthy subjects.95 They found a vaccine-induced increase in cfPWV and a decrease in AIx, which were associated with increases in C-reactive protein and interleukin-6 (biochemical markers of inflammation). Inflammation also causes vascular calcification and release of matrix metalloproteinases, which contribute to vascular remodeling.96
Hypertension
Smoking increases blood pressure and the risk for hypertension.97, 98, 99, 100 Studies have shown that in subjects with hypertension, arterial stiffness is increased compared with normotensive subjects of the same age.101, 102, 103 This increased stiffness leads to elevated systolic pressure and a widened pulse pressure which, in turn, leads to further tension on the arterial wall generating a positive feedback effect.104, 105 The wall tension induces mechanical vessel wall damage, vascular hypertrophy, increased collagen and calcium deposition, smooth muscle cell restructuring and extracellular matrix deposition, which leads to increases in arterial stiffness and end-organ damage, such as left ventricular hypertrophy.105, 106, 107, 108, 109, 110 Treatment of hypertension may help to decrease arterial stiffness; a number of studies have found that antihypertensive therapy can lead to decreased arterial stiffness in addition to blood pressure lowering.111, 112, 113, 114, 115 However, smoking has been found to blunt the arterial stiffness-lowering effect of antihypertensive therapy. Matsui et al.113 treated hypertensive smokers and nonsmokers with amlodipine for 6 months and noted that reduction in baPWV in smokers only reached the level of nonsmokers after 6 months of treatment. Therefore, it seems that smoking and hypertension exert synergistic effects on arterial stiffness, potentially through oxidative stress and the production of reactive oxygen species leading to degradation of NO, and by changes in production of endothelin-1 and prostacyclins.14, 106, 116, 117, 118, 119, 120, 121
Endothelial dysfunction
As discussed above, numerous previous studies have shown that smoking causes significant endothelial dysfunction as assessed by FMD.5, 6, 61, 62, 64 However, it is important to note that FMD has been previously shown to be significantly associated with arterial stiffness under a number of conditions and disease states.122, 123, 124, 125, 126 Wright et al.127 noted that this association was weak and suggested that measurements of arterial stiffness should not replace FMD. However, they used neither AIx nor cfPWV (the ‘gold standard’) as arterial stiffness measurements in their study. Furthermore, Yufu et al.40 found FMD to be strongly correlated with baPWV in healthy smokers (P<0.005). In addition, Siasos et al.90, 128 showed that smokers under L-arginine treatment have an increase in FMD concurrently with decreases in both AIx and cfPWV.
Blood markers
A number of blood markers carry potential to monitor the progression of arterial stiffness in smokers. Smokers have significantly higher plasma levels of homocysteine, fibrinogen, C-reactive protein, interleukin-6, triglycerides, low-density lipoprotein, as well as decreased high-density lipoprotein and apolipoprotein A1 levels.129, 130, 131 Many of these blood markers and others (such as lycopene, uric acid, adiponectin) have been related to increased arterial stiffness and may also have value in monitoring arterial stiffness in smokers and former smokers.39, 94, 95, 132, 133, 134, 135, 136, 137 However, it has been suggested that the use of multiple blood markers is of little prognostic value,96, 138 and that even the use of single markers such as C-reactive protein or homocysteine did not improve prediction of outcome using the Framingham risk score.138 Perhaps simply measuring cfPWV and AIx may prove the simplest and most effective method to assess and monitor vascular health in smokers.
Limitations
This review is limited by the comparability of the studies included. Protocols and methods were not standardized; the time of day the study took place, whether the subjects fasted or refrained from alcohol and caffeine before the study were different between protocols, and some did not report this information. Furthermore, the age groups of subjects, duration and intensity of smoking, body mass index, renal function, menopausal status, lipid profile, medication use, including statin use, were not uniform between studies or subject groups. These factors were also often not reported or corrected for; clearly, future studies investigating the effect of smoking on arterial stiffness should recruit homogeneous populations. The majority of the identified studies are cross-sectional; however, population-based longitudinal studies would be better suited to study the effect of smoking on arterial stiffness and to better track the progression of improvement in arterial stiffness after smoking cessation. Perhaps the most pertinent limitation of the included studies is the different methods used to obtain information about arterial stiffness. cfPWV is the ‘gold standard’ for measurement of arterial stiffness, and cfPWV and AIx have the greatest amount of evidence to support their predictive value for cardiovascular events.16, 17, 18, 19, 20, 21, 22 Therefore, other parameters of arterial stiffness, such as local distensibility or compliance, may not be as valuable to assess the vascular effects of smoking and are not useful clinical parameters. These should be used sparingly compared with cfPWV and AIx. Owing to these limitations of the included studies, a meta-analysis aiming to quantify the overall magnitude of the effect of smoking on arterial stiffness could not be conducted. However, the results of the included studies are comparable and point to smoking having a detrimental effect on arterial stiffness.
Clinical implications
Increased arterial stiffness is associated with increased risk of cardiovascular complications,16, 17, 18, 19, 20, 21, 22 and a strong correlation between arterial stiffness and the development of atherosclerosis at various sites in arteries has been noted.139, 140 Therefore, measuring arterial stiffness can be a useful clinical tool for disease progression and monitoring of treatment efficacy, as recommended by the European Network for Non-invasive Investigation of Large Arteries15 and the 2007 European guidelines for the management of arterial hypertension.16 The use of arterial stiffness measurements may be particularly useful in assessing endothelial damage induced by certain cardiovascular risk factors, such as cigarette smoking. cfPWV, the ‘gold standard’ for the assessment of aortic stiffness,15, 16 has been used in numerous longitudinal studies, and has the greatest amount of epidemiological evidence to support its predictive value for cardiovascular events in the general and diseased populations.15, 16, 17, 18, 19, 20, 141, 142, 143, 144, 145 Therefore, cfPWV should be the first choice in the assessment of arterial stiffness.
At present, 28% of European adults smoke,146 19% of the Canadian population aged 15 years and older are smokers,2 and 21% of adults in the United States smoke;147 this represents significant proportions of these populations. Moreover, although the general population smoking rates have been declining recently, young people seem to be picking up the habit; 20% of the youth smoked in Europe between the years 1999 and 2001, that number increased to 24% between 2002 and 2005.146 A similar trend exists in Canada; the percentage of smokers in individuals aged 20–24 years is now 28%, up from 24% in 2007.2 It is clear that smoking still represents a significant public health problem in North America and Europe, and the use of arterial stiffness assessments to monitor cardiovascular damage may be valuable from a clinical point of view. In addition, arterial stiffness measurements can also be used to assess the effectiveness of smoking cessation therapies, as well as the direct effect of the smoking cessation medications on arterial stiffness.
At present, there are several pharmacological options for smoking cessation available, such as nicotine replacement therapy (NRT),148, 149 which can help people increase their chances of successfully stopping smoking by 50–70%.148, 150 The market for over-the-counter nicotine gum, which is the most popular form of NRT, had annual sales of more than $300 million at the end of September 2008 in the United States (http://us.infores.com). Although the use of nicotine gum is approved by the Food and Drug Administration and widely advertised, there is no evidence in the literature regarding its direct effect on arterial stiffness. However, it has been shown that administration of nicotine can increase arterial stiffness acutely.55, 151 Therefore, it is possible (although not yet studied) that NRT increases arterial stiffness for the duration of therapy. It is also possible that non-NRT smoking cessation options may not have these effects and thus may be more beneficial. However, these effects of NRT and non-NRT smoking options have yet to be investigated; future research needs to focus on the extent to which smoking cessation therapies (NRT and non-NRT) can lead to stabilization or even reversal of arterial stiffness.
Conclusions
To our knowledge, this is the first systematic review on the effect of smoking on arterial stiffness. We presented results obtained from 39 studies evaluating the effect of acute, chronic and passive smoking on arterial stiffness, and the reversibility of arterial stiffness after smoking cessation. Acute, chronic and passive smoking all have a detrimental effect on arterial stiffness, although the role of chronic smoking in increasing arterial stiffness is slightly more controversial. Chronic smoking was also shown to have a role in sensitizing arterial response to acute smoking. However, whether arterial stiffness is reversed after smoking cessation, and the timeline in which this occurs could not be determined from the available literature. Long-term longitudinal studies using the best recognized parameters of arterial stiffness (cfPWV and AIx) are required to clarify this issue. Furthermore, with the large number of pharmacological options for smoking cessation available, it will become important to identify the direct effect of these medications on arterial stiffness.
References
Mokdad AH, Marks JS, Stroup DF, Gerberding JL . Actual causes of death in the United States, 2000. JAMA 2004; 291: 1238–1245.
http://www.hc-sc.gc.ca/hl-vs/tobac-tabac/research-recherche/stat/ctums-esutc_2006-eng.php, 2008.
www.heartstats.org, 2009.
Cacciola RR, Guarino F, Polosa R . Relevance of endothelial-haemostatic dysfunction in cigarette smoking. Curr Med Chem 2007; 14: 1887–1892.
Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, Deanfield JE . Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med 1996; 334: 150–154.
Esen AM, Barutcu I, Acar M, Degirmenci B, Kaya D, Turkmen M, Melek M, Onrat E, Esen OB, Kirma C . Effect of smoking on endothelial function and wall thickness of brachial artery. Circ J 2004; 68: 1123–1126.
Brouwers MC, Reesink KD, van Greevenbroek MM, Meinders JM, van der Kallen CJ, Schaper N, Hoeks AP, Stehouwer CD . Increased arterial stiffness in familial combined hyperlipidemia. J Hypertens 2009; 27: 1009–1016.
Brinkley TE, Nicklas BJ, Kanaya AM, Satterfield S, Lakatta EG, Simonsick EM, Sutton-Tyrrell K, Kritchevsky SB . Plasma oxidized low-density lipoprotein levels and arterial stiffness in older adults: the health, aging, and body composition study. Hypertension 2009; 53: 846–852.
Heffernan KS, Karas RH, Kuvin JT, Jae SY, Vieira VJ, Fernhall B . Carotid artery stiffness, high-density lipoprotein cholesterol and inflammation in men with pre-hypertension. J Hum Hypertens 2009; 23: 590–596.
Miyai N, Arita M, Miyashita K, Morioka I, Takeda S . The influence of obesity and metabolic risk variables on brachial-ankle pulse wave velocity in healthy adolescents. J Hum Hypertens 2009; 23: 444–450.
Mule G, Cottone S, Cusimano P, Incalcaterra F, Giandalia M, Costanzo M, Nardi E, Palermo A, Geraci C, Costa R, Cerasola G . Inverse relationship between ambulatory arterial stiffness index and glomerular filtration rate in arterial hypertension. Am J Hypertens 2008; 21: 35–40.
Le NA, Brown WV, Davis WW, Herrington DM, Mosca L, Homma S, Eggleston B, Willens HJ, Raines JK . Comparison of the relation of triglyceride-rich lipoproteins and muscular artery compliance in healthy women versus healthy men. Am J Cardiol 2005; 95: 1049–1054.
Sierksma A, Lebrun CE, van der Schouw YT, Grobbee DE, Lamberts SW, Hendriks HF, Bots ML . Alcohol consumption in relation to aortic stiffness and aortic wave reflections: a cross-sectional study in healthy postmenopausal women. Arterioscler Thromb Vasc Biol 2004; 24: 342–348.
Scallan C, Doonan RJ, Daskalopoulou SS . The combined effect of hypertension and smoking on arterial stiffness. Clin Exp Hypertens 2009 (in press).
Laurent S, Cockcroft J, Van BL, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27: 2588–2605.
Mancia G, De BG, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A, Vahanian A, Camm J, De CR, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Kjeldsen SE, Erdine S, Narkiewicz K, Kiowski W, gabiti-Rosei E, Ambrosioni E, Cifkova R, Dominiczak A, Fagard R, Heagerty AM, Laurent S, Lindholm LH, Mancia G, Manolis A, Nilsson PM, Redon J, Schmieder RE, Struijker-Boudier HA, Viigimaa M, Filippatos G, Adamopoulos S, gabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Kiowski W, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O’Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van ZP, Viigimaa M, Waeber B, Williams B, Zamorano JL, The task force for the management of arterial hypertension of the European Society of Hypertension, The task force for the management of arterial hypertension of the European Society of Cardiology. 2007 Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2007; 28: 1462–1536.
Blacher J, Asmar R, Djane S, London GM, Safar ME . Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension 1999; 33: 1111–1117.
Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A . Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001; 37: 1236–1241.
Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S . Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension 2002; 39: 10–15.
Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J . Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 2006; 113: 664–670.
Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM . Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int 2003; 63: 1852–1860.
Weber T, Auer J, O’Rourke MF, Kvas E, Lassnig E, Lamm G, Stark N, Rammer M, Eber B . Increased arterial wave reflections predict severe cardiovascular events in patients undergoing percutaneous coronary interventions. Eur Heart J 2005; 26: 2657–2663.
Lemogoum D, Van BL, Leeman M, Degaute JP, van de BP . Ethnic differences in arterial stiffness and wave reflections after cigarette smoking. J Hypertens 2006; 24: 683–689.
Mahmud A, Feely J . Effect of smoking on arterial stiffness and pulse pressure amplification. Hypertension 2003; 41: 183–187.
Berlin I, Cournot A, Renout P, Duchier J, Safar M . Peripheral haemodynamic effects of smoking in habitual smokers. A methodological study. Eur J Clin Pharmacol 1990; 38: 57–60.
Vlachopoulos C, Alexopoulos N, Panagiotakos D, O’Rourke MF, Stefanadis C . Cigar smoking has an acute detrimental effect on arterial stiffness. Am J Hypertens 2004; 17: 299–303.
Kim JW, Park CG, Hong SJ, Park SM, Rha SW, Seo HS, Oh DJ, Rho YM . Acute and chronic effects of cigarette smoking on arterial stiffness. Blood Press 2005; 14: 80–85.
Rhee MY, Na SH, Kim YK, Lee MM, Kim HY . Acute effects of cigarette smoking on arterial stiffness and blood pressure in male smokers with hypertension. Am J Hypertens 2007; 20: 637–641.
Failla M, Grappiolo A, Carugo S, Calchera I, Giannattasio C, Mancia G . Effects of cigarette smoking on carotid and radial artery distensibility. J Hypertens 1997; 15: 1659–1664.
Giannattasio C, Mangoni AA, Stella ML, Carugo S, Grassi G, Mancia G . Acute effects of smoking on radial artery compliance in humans. J Hypertens 1994; 12: 691–696.
Zamir Z, Mahmud A, Feely J . Acute haemodynamic effects of cigarette smoking in healthy young subjects. Ir J Med Sci 2006; 175: 20–23.
Kool MJ, Hoeks AP, Struijker Boudier HA, Reneman RS, Van Bortel LM . Short- and long-term effects of smoking on arterial wall properties in habitual smokers. J Am Coll Cardiol 1993; 22: 1881–1886.
Stefanadis C, Tsiamis E, Vlachopoulos C, Stratos C, Toutouzas K, Pitsavos C, Marakas S, Boudoulas H, Toutouzas P . Unfavorable effect of smoking on the elastic properties of the human aorta. Circulation 1997; 95: 31–38.
Stefanadis C, Vlachopoulos C, Tsiamis E, Diamantopoulos L, Toutouzas K, Giatrakos N, Vaina S, Tsekoura D, Toutouzas P . Unfavorable effects of passive smoking on aortic function in men. Ann Intern Med 1998; 128: 426–434.
Swampillai J, Rakebrandt F, Morris K, Jones CJ, Fraser AG . Acute effects of caffeine and tobacco on arterial function and wave travel. Eur J Clin Invest 2006; 36: 844–849.
Levenson J, Simon AC, Cambien FA, Beretti C . Cigarette smoking and hypertension. Factors independently associated with blood hyperviscosity and arterial rigidity. Arteriosclerosis 1987; 7: 572–577.
Binder S, Navratil K, Halek J . Chronic smoking and its effect on arterial stiffness. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2008; 152: 299–302.
Nakanishi N, Suzuki K, Kawashimo H, Nakamura K, Tatara K . Risk factors for the incidence of aortic stiffness by serial aortic pulse wave velocity measurement in middle-aged Japanese men. Health Prevent Med 1998; 3: 168–174.
Nguyen QM, Srinivasan SR, Xu JH, Chen W, Berenson GS . Racial (black-white) divergence in the association between adiponectin and arterial stiffness in asymptomatic young adults: the Bogalusa heart study. Am J Hypertens 2008; 21: 553–557.
Yufu K, Takahashi N, Hara M, Saikawa T, Yoshimatsu H . Measurement of the brachial-ankle pulse wave velocity and flow-mediated dilatation in young, healthy smokers. Hypertens Res 2007; 30: 607–612.
Filipovsky J, Ticha M, Cifkova R, Lanska V, Stastna V, Roucka P . Large artery stiffness and pulse wave reflection: results of a population-based study. Blood Press 2005; 14: 45–52.
Rehill N, Beck CR, Yeo KR, Yeo WW . The effect of chronic tobacco smoking on arterial stiffness. Br J Clin Pharmacol 2006; 61: 767–773.
Taquet A, Bonithon-Kopp C, Simon A, Levenson J, Scarabin Y, Malmejac A, Ducimetiere P, Guize L . Relations of cardiovascular risk factors to aortic pulse wave velocity in asymptomatic middle-aged women. Eur J Epidemiol 1993; 9: 298–306.
van Trijp MJ, Bos WJ, Uiterwaal CS, Oren A, Vos LE, Grobbee DE, Bots ML . Determinants of augmentation index in young men: the ARYA study. Eur J Clin Invest 2004; 34: 825–830.
Fennessy F, Casey RG, Bouchier-Hayes D . Peripheral and central arterial haemodynamic interactions are early abnormalities in young male cigarette smokers. Eur J Vasc Endovasc Surg 2003; 25: 152–158.
Tomiyama H, Yamazaki M, Sagawa Y, Teraoka K, Shirota T, Miyawaki Y, Yamashina A . Synergistic effect of smoking and blood pressure on augmentation index in men, but not in women. Hypertens Res 2009; 32: 122–126.
Liang YL, Shiel LM, Teede H, Kotsopoulos D, McNeil J, Cameron JD, McGrath BP . Effects of blood pressure, smoking, and their interaction on carotid artery structure and function. Hypertension 2001; 37: 6–11.
Jonason T, Henrikssen E, Kangro T, Nilsson H, Vessby B, Ringqvist I . Stiffness of the common carotid artery in healthy 50-year-old subjects. Clin Physiol 1997; 17: 569–577.
Sonesson B, Ahlgren AR, Lazer L, Lanne T . Does long-term smoking affect aortic stiffness more in women than in men? Clin Physiol 1997; 17: 439–447.
Terenzi T, Gallagher D, De MR . Smokers exhibit an altered Doppler analog waveform during peripheral arterial examination. J Manipulative Physiol Ther 1995; 18: 211–218.
Sassalos K, Vlachopoulos C, Alexopoulos N, Gialernios T, Aznaouridis K, Stefanadis C . The acute and chronic effect of cigarette smoking on the elastic properties of the ascending aorta in healthy male subjects. Hellenic J Cardiol 2006; 47: 263–268.
Levent E, Ozyurek AR, Ulger Z . Evaluation of aortic stiffness in tobacco-smoking adolescents. J Adolesc Health 2004; 34: 339–343.
McVeigh GE, Morgan DJ, Finkelstein SM, Lemay LA, Cohn JN . Vascular abnormalities associated with long-term cigarette smoking identified by arterial waveform analysis. Am J Med 1997; 102: 227–231.
Wollersheim H, Firestone G, Fronek A . Selective increase in popliteal artery wall stiffness in long-term smokers. J Hypertens Suppl 1993; 11: S84–S85.
Argacha JF, Adamopoulos D, Gujic M, Fontaine D, Amyai N, Berkenboom G, van de BP . Acute effects of passive smoking on peripheral vascular function. Hypertension 2008; 51: 1506–1511.
Mahmud A, Feely J . Effects of passive smoking on blood pressure and aortic pressure waveform in healthy young adults—influence of gender. Br J Clin Pharmacol 2004; 57: 37–43.
Mack WJ, Islam T, Lee Z, Selzer RH, Hodis HN . Environmental tobacco smoke and carotid arterial stiffness. Prev Med 2003; 37: 148–154.
Oren S, Isakov I, Golzman B, Kogan J, Turkot S, Peled R, Yosefy C . The influence of smoking cessation on hemodynamics and arterial compliance. Angiology 2006; 57: 564–568.
Polonia J, Barbosa L, Silva JA, Rosas M . Improvement of aortic reflection wave responses 6 months after stopping smoking: a prospective study. Blood Press Monit 2009; 14: 69–75.
van den Berkmortel FW, Wollersheim H, van LH, Smilde TJ, den AJ, Thien T . Two years of smoking cessation does not reduce arterial wall thickness and stiffness. Neth J Med 2004; 62: 235–241.
Karatzi K, Papamichael C, Karatzis E, Papaioannou TG, Stamatelopoulos K, Zakopoulos NA, Zampelas A, Lekakis J . Acute smoke-induced endothelial dysfunction is more prolonged in smokers than in non-smokers. Int J Cardiol 2007; 120: 404–406.
Gordon JL, Lavoie KL, Arsenault A, Ditto B, Bacon SL . Health behaviors and endothelial function. J Behav Med 2008; 31: 5–21.
Hayashi K, Handa H, Nagasawa S, Okumura A, Moritake K . Stiffness and elastic behavior of human intracranial and extracranial arteries. J Biomech 1980; 13: 175–184.
Raitakari OT, Adams MR, McCredie RJ, Griffiths KA, Celermajer DS . Arterial endothelial dysfunction related to passive smoking is potentially reversible in healthy young adults. Ann Intern Med 1999; 130: 578–581.
Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE . Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation 1993; 88: 2149–2155.
Raitakari OT, Adams MR, McCredie RJ, Griffiths KA, Celermajer DS . Arterial endothelial dysfunction related to passive smoking is potentially reversible in healthy young adults. Ann Intern Med 1999; 130: 578–581.
Hammond EC . Smoking in relation to the death rates of one million men and women. Natl Cancer Inst Monogr 1966; 19: 127–204.
Hammond EC, HORN D . Smoking and death rates; report on forty-four months of follow-up of 187,783 men. I. Total mortality. J Am Med Assoc 1958; 166: 1159–1172.
Best EWR, Josie GH, Walker CB . A Canadian study of mortality in relation to smoking habits. Can J Public Health 1961; 52: 99–106.
Jatoi NA, Jerrard-Dunne P, Feely J, Mahmud A . Impact of smoking and smoking cessation on arterial stiffness and aortic wave reflection in hypertension. Hypertension 2007; 49: 981–985.
Barnoya J, Glantz SA . Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation 2005; 111: 2684–2698.
Chelland CS, Moffatt RJ, Stamford BA . Smoking and smoking cessation—the relationship between cardiovascular disease and lipoprotein metabolism: a review. Atherosclerosis 2008; 201: 225–235.
Riccioni G . Statins and carotid intima-media thickness reduction: an up-to-date review. Curr Med Chem 2009; 16: 1799–1805.
Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, Clegg LX . Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol 1997; 146: 483–494.
Daskalopoulou SS . When statin therapy stops: implications for the patient. Curr Opin Cardiol 2009; 24: 454–460.
Maki-Petaja KM, Wilkinson IB . Anti-inflammatory drugs and statins for arterial stiffness reduction. Curr Pharm Des 2009; 15: 290–303.
Orth SR . Effects of smoking on systemic and intrarenal hemodynamics: influence on renal function. J Am Soc Nephrol 2004; 15: S58–S63.
Orth SR, Ritz E . The renal risks of smoking: an update. Curr Opin Nephrol Hypertens 2002; 11: 483–488.
Safar ME, London GM, Plante GE . Arterial stiffness and kidney function. Hypertension 2004; 43: 163–168.
Chiolero A, Faeh D, Paccaud F, Cornuz J . Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr 2008; 87: 801–809.
Gordin D, Ronnback M, Forsblom C, Makinen V, Saraheimo M, Groop PH . Glucose variability, blood pressure and arterial stiffness in type 1 diabetes. Diabetes Res Clin Pract 2008; 80: e4–e7.
Gedikli O, Ozturk S, Yilmaz H, Baykan M, Kiris A, Durmus I, Karaman K, Karahan C, Celik S . Low total antioxidative capacity levels are associated with augmentation index but not pulse-wave velocity. Heart Vessels 2009; 24: 366–370.
Cameron JD, Cruickshank JK . Glucose, insulin, diabetes and mechanisms of arterial dysfunction. Clin Exp Pharmacol Physiol 2007; 34: 677–682.
Rahman MM, Laher I . Structural and functional alteration of blood vessels caused by cigarette smoking: an overview of molecular mechanisms. Curr Vasc Pharmacol 2007; 5: 276–292.
Peluffo G, Calcerrada P, Piacenza L, Pizzano N, Radi R . Superoxide-mediated inactivation of nitric oxide and peroxynitrite formation by tobacco smoke in vascular endothelium: studies in cultured cells and smokers. Am J Physiol Heart Circ Physiol 2009; 296: H1781–H1792.
Valavanidis A, Vlachogianni T, Fiotakis K . Tobacco smoke: involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int J Environ Res Public Health 2009; 6: 445–462.
Takajo Y, Ikeda H, Haramaki N, Murohara T, Imaizumi T . Augmented oxidative stress of platelets in chronic smokers. Mechanisms of impaired platelet-derived nitric oxide bioactivity and augmented platelet aggregability. J Am Coll Cardiol 2001; 38: 1320–1327.
Delles C, Zimmerli LU, McGrane DJ, Koh-Tan CH, Pathi VL, McKay AJ, Steedman T, Dargie HJ, Hamilton CA, Dominiczak AF . Vascular stiffness is related to superoxide generation in the vessel wall. J Hypertens 2008; 26: 946–955.
Mayer JO, Filipovsky J, Pesta M, Cifkova R, Dolejsova M, Simon J . The interaction of endothelial nitric oxide synthase polymorphism and current smoking in terms of increased arterial stiffness. Physiol Res 2009 (e-pub ahead of print).
Siasos G, Tousoulis D, Vlachopoulos C, Antoniades C, Stefanadi E, Ioakeimidis N, Zisimos K, Siasou Z, Papavassiliou AG, Stefanadis C . The impact of oral L-arginine supplementation on acute smoking-induced endothelial injury and arterial performance. Am J Hypertens 2009; 22: 586–592.
Arnson Y, Shoenfeld Y, Amital H . Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun 2009; 34: J258–J265.
Kals J, Kampus P, Kals M, Pulges A, Teesalu R, Zilmer K, Kullisaar T, Salum T, Eha J, Zilmer M . Inflammation and oxidative stress are associated differently with endothelial function and arterial stiffness in healthy subjects and in patients with atherosclerosis. Scand J Clin Lab Invest 2008; 68: 594–601.
Cecelja M, Chowienczyk P . Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension 2009; 54: 1328–1336.
Schumacher W, Cockcroft J, Timpson NJ, McEniery CM, Gallacher J, Rumley A, Lowe G, Smith GD, Wilkinson IB, Ben-Shlomo Y . Association between C-reactive protein genotype, circulating levels, and aortic pulse wave velocity. Hypertension 2009; 53: 150–157.
Vlachopoulos C, Dima I, Aznaouridis K, Vasiliadou C, Loakeimidis N, Aggeli C, Toutouza M, Stefanadis C . Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation 2005; 112: 2193–2200.
Boutouyrie P, Laurent S, Briet M . Importance of arterial stiffness as cardiovascular risk factor for future development of new type of drugs. Fundam Clin Pharmacol 2008; 22: 241–246.
Minami J, Ishimitsu T, Ohrui M, Matsuoka H . Association of smoking with aortic wave reflection and central systolic pressure and metabolic syndrome in normotensive Japanese men. Am J Hypertens 2009; 22: 617–623.
Pardell H, Rodicio JL . High blood pressure, smoking and cardiovascular risk. J Hypertens 2005; 23: 219–221.
Benowitz NL . Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addition. N Engl J Med 1988; 319: 1318–1330.
Rempher KJ . Cardiovascular sequelae of tobacco smoking. Crit Care Nurs Clin North Am 2006; 18: 13–20, xi.
Asmar R, Benetos A, London G, Hugue C, Weiss Y, Topouchian J, Laloux B, Safar M . Aortic distensibility in normotensive, untreated and treated hypertensive patients. Blood Press 1995; 4: 48–54.
Hasegawa M, Nagao K, Kinoshita Y, Rodbard D, Asahina A . Increased pulse wave velocity and shortened pulse wave transmission time in hypertension and aging. Cardiology 1997; 88: 147–151.
McEniery CM, Wilkinson IB, Avolio AP . Age, hypertension and arterial function. Clin Exp Pharmacol Physiol 2007; 34: 665–671.
Nichols W, O’Rourke M . McDonald's Blood Flow in Arteries, 4th edn. Edward Arnold: London, 1998. Ref type: Generic.
Dart AM, Kingwell BA . Pulse pressure—a review of mechanisms and clinical relevance. J Am Coll Cardiol 2001; 37: 975–984.
Schiffrin EL . Small artery remodeling in hypertension: can it be corrected? Am J Med Sci 2001; 322: 7–11.
Laurent S, Boutouyrie P . Recent advances in arterial stiffness and wave reflection in human hypertension. Hypertension 2007; 49: 1202–1206.
Toprak A, Reddy J, Chen W, Srinivasan S, Berenson G . Relation of pulse pressure and arterial stiffness to concentric left ventricular hypertrophy in young men (from the Bogalusa Heart Study). Am J Cardiol 2009; 103: 978–984.
Rodilla E, Costa JA, Perez-Lahiguera F, Gonzalez C, Pascual JM . Relationship between increased arterial stiffness and other markers of target organ damage. Med Clin (Barc) 2009 (e-pub ahead of print).
Graham MR, Evans P, Davies B, Baker JS . Arterial pulse wave velocity, inflammatory markers, pathological GH and IGF states, cardiovascular and cerebrovascular disease. Vasc Health Risk Manag 2008; 4: 1361–1371.
Sasamura H, Kitamura Y, Nakamura M, Ryuzaki M, Saruta T . Effects of the angiotensin receptor blocker candesartan on arterial stiffness and markers of extracellular matrix metabolism in patients with essential hypertension. Clin Exp Hypertens 2006; 28: 511–520.
Hirata K, Vlachopoulos C, Adji A, O’Rourke MF . Benefits from angiotensin-converting enzyme inhibitor ‘beyond blood pressure lowering’: beyond blood pressure or beyond the brachial artery? J Hypertens 2005; 23: 551–556.
Matsui Y, Kario K, Ishikawa J, Hoshide S, Eguchi K, Shimada K . Smoking and antihypertensive medication: interaction between blood pressure reduction and arterial stiffness. Hypertens Res 2005; 28: 631–638.
Ferguson JM, Minas J, Siapantas S, Komesaroff PA, Sudhir K . Effects of a fixed-dose ACE inhibitor-diuretic combination on ambulatory blood pressure and arterial properties in isolated systolic hypertension. J Cardiovasc Pharmacol 2008; 51: 590–595.
Gosse P, Coulon P, Papaioannou G, Lemetayer P . Long-term influence of antihypertensive treatment on arterial stiffness assessed by ambulatory measurement of the QKD interval. Hypertens Res 2009; 32: 265–269.
Tschudi MR, Mesaros S, Luscher TF, Malinski T . Direct in situ measurement of nitric oxide in mesenteric resistance arteries. Increased decomposition by superoxide in hypertension. Hypertension 1996; 27: 32–35.
Feihl F, Liaudet L, Levy BI, Waeber B . Hypertension and microvascular remodelling. Cardiovasc Res 2008; 78: 274–285.
Landmesser U, Drexler H . Endothelial function and hypertension. Curr Opin Cardiol 2007; 22: 316–320.
Jeremy JY, Mikhailidis DP, Dandona P . Cigarette smoke extracts, but not nicotine, inhibit prostacyclin (PGI2) synthesis in human, rabbit and rat vascular tissue. Prostaglandins Leukot Med 1985; 19: 261–270.
Nakayama T, Hironaga T, Ishima H, Maruyama T, Masubuchi Y, Kokubun S . The prostacyclin analogue beraprost sodium prevents development of arterial stiffness in elderly patients with cerebral infarction. Prostaglandins Leukot Essent Fatty Acids 2004; 70: 491–494.
Yildiz L, Akcay F, Kaynar H, Bakan N . Increased plasma endothelin-1 in heavy and light smokers. Clin Chem 1996; 42: 483–484.
Soltesz P, Der H, Kerekes G, Szodoray P, Szucs G, Danko K, Shoenfeld Y, Szegedi G, Szekanecz Z . A comparative study of arterial stiffness, flow-mediated vasodilation of the brachial artery, and the thickness of the carotid artery intima-media in patients with systemic autoimmune diseases. Clin Rheumatol 2009; 28: 655–662.
Kollias GE, Stamatelopoulos KS, Papaioannou TG, Zakopoulos NA, Alevizaki M, Alexopoulos GP, Kontoyannis DA, Karga H, Koroboki E, Lekakis JP, Papamichael CM . Diurnal variation of endothelial function and arterial stiffness in hypertension. J Hum Hypertens 2009; 23: 597–604.
Kopec G, Podolec P, Podolec J, Rubis P, Zmudka K, Tracz W . Atherosclerosis progression affects the relationship between endothelial function and aortic stiffness. Atherosclerosis 2009; 204: 250–254.
Haller MJ, Stein JM, Shuster JJ, Theriaque D, Samyn MM, Pepine C, Silverstein JH . Pediatric Atorvastatin in Diabetes Trial (PADIT): a pilot study to determine the effect of atorvastatin on arterial stiffness and endothelial function in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2009; 22: 65–68.
Kohler M, Craig S, Nicoll D, Leeson P, Davies RJ, Stradling JR . Endothelial function and arterial stiffness in minimally symptomatic obstructive sleep apnea. Am J Respir Crit Care Med 2008; 178: 984–988.
Wright CI, Scholten HJ, Schilder JC, Elsen BM, Hanselaar W, Kroner CI, Draijer R, Kastelein JJ, Stok W, Karemaker J, de GE . Arterial stiffness, endothelial function and microcirculatory reactivity in healthy young males. Clin Physiol Funct Imaging 2008; 28: 299–306.
Siasos G, Tousoulis D, Vlachopoulos C, Antoniades C, Stefanadi E, Ioakeimidis N, Andreou I, Zisimos K, Papavassiliou AG, Stefanadis C . Short-term treatment with L-arginine prevents the smoking-induced impairment of endothelial function and vascular elastic properties in young individuals. Int J Cardiol 2008; 126: 394–399.
Berlin I . Smoking-induced metabolic disorders: a review. Diabetes Metab 2008; 34: 307–314.
Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF . Systemic effects of smoking. Chest 2007; 131: 1557–1566.
Jefferis BJ, Lowe GD, Welsh P, Rumley A, Lawlor DA, Ebrahim S, Carson C, Doig M, Feyerabend C, McMeekin L, Wannamethee SG, Cook DG, Whincup PH . Secondhand smoke (SHS) exposure is associated with circulating markers of inflammation and endothelial function in adult men and women. Atherosclerosis 2009; 208: 550–556.
Kim OY, Yoe HY, Kim HJ, Park JY, Kim JY, Lee SH, Lee JH, Lee KP, Jang Y, Lee JH . Independent inverse relationship between serum lycopene concentration and arterial stiffness. Atherosclerosis 2009; 208: 581–586.
Tsioufis C, Dimitriadis K, Selima M, Thomopoulos C, Mihas C, Skiadas I, Tousoulis D, Stefanadis C, Kallikazaros I . Low-grade inflammation and hypoadiponectinaemia have an additive detrimental effect on aortic stiffness in essential hypertensive patients. Eur Heart J 2007; 28: 1162–1169.
Ruan L, Chen W, Srinivasan SR, Xu J, Sun M, Toprak A, Berenson GS . Relation of plasma homocysteine to arterial stiffness in black and white young adults (from the Bogalusa Heart Study). Am J Cardiol 2009; 103: 985–988.
Levy D, Hwang SJ, Kayalar A, Benjamin EJ, Vasan RS, Parise H, Larson MG, Wang TJ, Selhub J, Jacques PF, Vita JA, Keyes MJ, Mitchell GF . Associations of plasma natriuretic peptide, adrenomedullin, and homocysteine levels with alterations in arterial stiffness: the Framingham Heart Study. Circulation 2007; 115: 3079–3085.
Vlachopoulos C, Pietri P, Aznaouridis K, Vyssoulis G, Vasiliadou C, Bratsas A, Tousoulis D, Xaplanteris P, Stefanadi E, Stefanadis C . Relationship of fibrinogen with arterial stiffness and wave reflections. J Hypertens 2007; 25: 2110–2116.
Vlachopoulos C, Pietri P, Aznaouridis K, Vyssoulis G, Vasiliadou C, Bratsas A, Tousoulis D, Xaplanteris P, Stefanadi E, Stefanadis C . Relationship of fibrinogen with arterial stiffness and wave reflections. J Hypertens 2007; 25: 2110–2116.
Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D’Agostino RB, Vasan RS . Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med 2006; 355: 2631–2639.
Maarek B, Simon AC, Levenson J, Pithois-Merli I, Bouthier J . Heterogeneity of the atherosclerotic process in systemic hypertension poorly controlled by drug treatment. Am J Cardiol 1987; 59: 414–417.
Wada T, Kodaira K, Fujishiro K, Maie K, Tsukiyama E, Fukumoto T, Uchida T, Yamazaki S . Correlation of ultrasound-measured common carotid artery stiffness with pathological findings. Arterioscler Thromb 1994; 14: 479–482.
Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM . Impact of aortic stiffness on survival in end-stage renal disease. Circulation 1999; 99: 2434–2439.
Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG . Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation 2002; 106: 2085–2090.
Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, Boutouyrie P . Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke 2003; 34: 1203–1206.
Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME . Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol 2001; 21: 2046–2050.
Shoji T, Emoto M, Shinohara K, Kakiya R, Tsujimoto Y, Kishimoto H, Ishimura E, Tabata T, Nishizawa Y . Diabetes mellitus, aortic stiffness, and cardiovascular mortality in end-stage renal disease. J Am Soc Nephrol 2001; 12: 2117–2124.
National Center for Health Statistics. National Health Interview Survey 2005; Series 10, No. 232. Page 9.
Eisenberg MJ, Filion KB, Yavin D, Belisle P, Mottillo S, Joseph L, Gervais A, O’Loughlin J, Paradis G, Rinfret S, Pilote L . Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ 2008; 179: 135–144.
Shiffman S, Cone EJ, Buchhalter AR, Henningfield JE, Rohay JM, Gitchell JG, Pinney JM, Chau T . Rapid absorption of nicotine from new nicotine gum formulations. Pharmacol Biochem Behav 2008; 91: 380–384.
Stead LF, Perera R, Bullen C, Mant D, Lancaster T . Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2008 (Article number CD000146).
Chung S, Yoon IY, Shin YK, Lee CH, Kim JW, Ahn HJ . Endothelial dysfunction and inflammatory reactions of elderly and middle-aged men with obstructive sleep apnea syndrome. Sleep Breath 2009; 13: 11–17.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Hypertension Research website
Rights and permissions
About this article
Cite this article
Doonan, R., Hausvater, A., Scallan, C. et al. The effect of smoking on arterial stiffness. Hypertens Res 33, 398–410 (2010). https://doi.org/10.1038/hr.2010.25
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2010.25
Keywords
This article is cited by
-
Effects of Smoking on Intima-Media Thickness of the Common Carotid Artery Using Ultrasonography
Artery Research (2024)
-
Arterial stiffness and hypertension
Clinical Hypertension (2023)
-
Chronic cigarette smoking is associated with increased arterial stiffness in men and women: evidence from a large population-based cohort
Clinical Research in Cardiology (2023)
-
Effect of Amlodipine/Nebivolol combination therapy on central BP and PWV compared to Amlodipine/Valsartan combination therapy
The Egyptian Heart Journal (2022)
-
The effect of smoking on quantification of aortic stiffness by ultrasound time-harmonic elastography
Scientific Reports (2022)