Abstract
This 12-week, multicenter, open-label study assessed the efficacy, pharmacokinetics and safety of a once-daily aliskiren in Japanese hypertensive patients with renal dysfunction. Patients (n=40, aged 20–80 years) with mean sitting diastolic blood pressure (msDBP) ⩾95 and <110 mm Hg and serum creatinine between ⩾1.3 and <3.0 mg per 100 ml in males or between ⩾1.2 and <3.0 mg per 100 ml in females were eligible. Patients began therapy with a once-daily morning oral dose of 75 mg of aliskiren. In patients with inadequate blood pressure control (msDBP ⩾90 or mean sitting systolic blood pressure [msSBP] ⩾140 mm Hg) and without safety concerns (serum potassium >5.5 mEq l–1 or an increase in serum creatinine ⩾20%), the aliskiren dose was increased to 150 mg and then to 300 mg in sequential steps starting from Week 2. Efficacy was assessed as change in msSBP/msDBP from baseline to the Week 8 endpoint (with the last observation carried forward). The mean reduction from baseline to Week 8 endpoint was 13.9±16.6 and 11.6±9.7 mm Hg for msSBP and msDBP, respectively. At the Week 8 endpoint, 65% patients had achieved blood pressure response (msDBP <90 or a 10 mm Hg decrease or msSBP <140 or a 20 mm Hg decrease) and 30% had achieved blood pressure control (msSBP <140 mm Hg and msDBP <90 mm Hg). Aliskiren was well tolerated with no new safety concerns in Japanese hypertensive patients with renal dysfunction.
Similar content being viewed by others
Introduction
Hypertension is a major modifiable risk factor for cardiovascular and renal disease that affects >25% of the adult population worldwide.1 Despite the high prevalence of hypertension in patients with chronic kidney disease (81.8%), only 65.9% receive antihypertensive therapy and, in those that do receive treatment, only 23.3% achieve blood pressure control (systolic blood pressure/diastolic blood pressure <130/80 mm Hg).2 Hence, optimal blood pressure management may improve clinical outcomes in these high-risk hypertensive patients, especially because some classes of antihypertensive drugs may offer renal protection independent of blood pressure lowering effect.3
The renin–angiotensin–aldosterone system (RAAS), a major regulator of blood pressure, is an important therapeutic target for antihypertensive therapy.4 Although angiotensin I-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are effective in controlling blood pressure, these antihypertensives do not completely suppress the RAAS, leading to a reactive rise in plasma renin activity. In addition, the use of these antihypertensive agents slows renal deterioration, but does not completely stop the progression of renal disease.5, 6, 7 Therefore, benefits in hypertensive patients with renal disease above and beyond that offered by ACEIs and ARBs may be achievable. Aliskiren, an oral direct renin inhibitor for treatment of hypertension, effectively reduces plasma renin activity, resulting in a more complete suppression of RAAS.8 Further, aliskiren as a once-daily oral treatment (up to 300 mg per day) has shown dose-dependent reductions in both systolic and diastolic blood pressure in patients with essential hypertension.9, 10
In Japan, adequate blood pressure control is achieved in <50% patients, and still fewer of those with co-morbidities such as diabetes mellitus.11 Drugs that target RAAS are being increasingly used.11 Although aliskiren has shown dose-dependent efficacy in Japanese patients with essential hypertension,12 there is limited data regarding direct renin inhibitors in the high-risk population of hypertensive patients with renal dysfunction. This was the first study to evaluate the safety and efficacy of aliskiren in Japanese hypertensive patients with renal dysfunction.
Methods
Study design
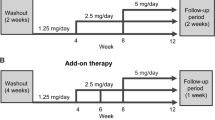
This was a 12-week, multicenter, single-arm, open-label study consisting of a 4-week placebo run-in period to wash out the effects of earlier antihypertensives, and an 8-week treatment period (Figure 1). The study was conducted according to the Declaration of Helsinki and in compliance with the Good Clinical Practice. This study was initiated only after obtaining institutional review board approval at each study center. Written informed consent was obtained from all patients.
Study design. *Optional dose titration for inadequately controlled blood pressure (msDBP ⩾90 mm Hg or msSBP ⩾140 mm Hg) and no safety concerns (serum potassium level over 5.5 mEq l–1 or an increase in serum creatinine ⩾20%). †Continued given that there was no change in drug or dosing regimen. BP, blood pressure; TPA, trough plasma aliskiren concentration.
Patients
Male or female Japanese outpatients (aged 20–80 years) with mild-to-moderate essential hypertension (mean sitting diastolic blood pressure [msDBP] ⩾95 and <110 mm Hg) and renal dysfunction (serum creatinine levels ⩾1.3 and <3.0 mg per 100 ml in males or ⩾1.2 and <3.0 mg per 100 ml in females) were included in the study. Patients were excluded if they had severe hypertension (msDBP ⩾110 and/or mean sitting systolic blood pressure [msSBP] ⩾180 mm Hg), secondary hypertension (such as renovascular hypertension except renoparenchymal hypertension), malignant hypertension, type 1 diabetes mellitus or type 2 diabetes mellitus with poor glucose control (glycosylated hemoglobin [HbA1c] >8% at the start of the run-in period), a history of cardiovascular disease or any condition (medical or surgical) that might affect the absorption, distribution, metabolism or excretion of the study drug. Women who were pregnant, nursing or those with child-bearing potential were excluded. Patients with a history of allergy or hypersensitivity to the study drug were also excluded.
Treatment
After a 4-week placebo run-in period, all eligible patients entered an 8-week optional dose-titration period during which they received aliskiren 75–300 mg once daily. As the Japanese Society of Hypertension guidelines for the management of hypertension13 recommend that treatment with drugs acting on RAAS should be initiated at the minimum dose when serum creatinine is ⩾2.0 mg per 100 ml, optional dose titration was used in stages to assess the safety of aliskiren in renal dysfunction. Eligible patients began therapy with a once-daily oral morning dose of 75 mg of aliskiren, except on the days of scheduled visits when aliskiren was administered after the completion of all assessments. The timing of food was not restricted and dosing could be pre- or post-prandial; however, on scheduled visit days, patients reported while fasting and without taking aliskiren and diuretics until the completion of all assessments. Patients who were on diuretics (other than potassium-sparing diuretics) before the initiation of the study continued to take them concurrently, as long as there was no change in the drug or the dosing regimen during the study. Concomitant use of other antihypertensive agents was not permitted from the start of the run-in period to end of treatment period.
For patients whose blood pressure was not adequately controlled with the initial dose (msDBP ⩾90 or msSBP ⩾140 mm Hg) and who had no safety concerns (serum potassium was not >5.5 mEq l–1 and serum creatinine had not increased by ⩾20% from baseline), aliskiren was up-titrated to 150 and then to 300 mg in sequential steps starting from Week 2.
Efficacy assessments
The primary efficacy outcome was the change in msSBP/msDBP from baseline (Week 0) to Week 8 (last observation carried forward). Blood pressure was measured three times at all scheduled visits (Weeks −4, −2, 0, 2, 4, 6 and 8), taken at 1- to 2-min intervals after resting for at least 5 min in the sitting position. The reported blood pressure for the visit was the average of all three measurements. Additional assessments included the following: (i) msDBP responder rate (defined as the proportion of patients with an absolute msDBP <90 or a ⩾10 mm Hg reduction from baseline to Week 8), (ii) msSBP responder rate (defined as the proportion of patients with an absolute msSBP <140 or a ⩾20 mm Hg reduction from baseline to Week 8), (iii) msDBP or msSBP responder rates and (iv) control rate (defined as the proportion of patients with msDBP <90 mm Hg and msSBP <140 mm Hg).
Pharmacokinetic assessments
Trough plasma aliskiren concentrations were measured at Weeks 2, 4 and 8 in the treatment period. Blood samples were drawn from a forearm vein using heparinized blood sample tubes and were centrifuged at 800 g for 15 min. Plasma was stored at −70 °C or below until measurement of the drug concentration. The concentration of aliskiren in plasma was determined by liquid chromatography/mass spectrometry. The lower limit of quantification was 0.5 ng ml–1.
Safety assessments
Safety and tolerability of aliskiren treatment in hypertensive patients with renal dysfunction was assessed by monitoring and recording adverse events, performing laboratory tests for hematology, blood chemistry and urinalysis, and monitoring vital signs, ECG and body weight. At all scheduled visits, adverse events were recorded and rated as mild, moderate or severe, and were assessed for relationship to study medication. Serious adverse events were reported up to 4 weeks after completion of the study. Standard laboratory tests were performed at baseline (Week 0) and at Weeks 4 and 8 in the treatment period. In addition, serum creatinine, blood urea nitrogen and serum electrolytes (sodium, potassium, chloride, calcium and phosphorous) were measured at Weeks 2 and 6 in the treatment period. The Cockcroft–Gault equation was used to calculate creatinine clearance (CCr).
Body weight was measured during all scheduled visits in the treatment period and a 12-lead ECG recording was obtained at Week −4 in the run-in period and at Week 8 of treatment. Change in orthostatic blood pressure (a decrease of ⩾20 mm Hg in systolic blood pressure or ⩾10 mm Hg in diastolic blood pressure when changing from a supine to a standing position) was also assessed. Treatment compliance was assessed at all visits using pill counts and information provided by the patients. Compliance was defined as taking the medication correctly on at least 70% of the days.
Data analysis
A target sample size of 30 patients was considered sufficient to evaluate the overall safety and tolerability of aliskiren treatment with 95% power to detect the incidence of adverse event (10%) in at least one patient. However, at least 38 patients had to be enrolled to allow for a 20% drop out rate. Efficacy and safety analyses were performed on all patients who received at least one dose of aliskiren. Patients with major protocol violations were excluded from the analyses. No statistical inference was performed in this study, and descriptive statistics were used for all efficacy and safety variables. The last observation carried forward method was used to assess the changes from baseline to Week 8 endpoint.
Results
Informed consent was obtained from 82 patients, and 78 patients entered the 4-week, placebo run-in period. Of these 78 patients, 38 discontinued the study before receiving the study drug. Of 40 patients who entered the treatment period, 35 completed aliskiren treatment and five discontinued treatment because of an adverse event or withdrew consent (Figure 2). All 40 patients were evaluable for efficacy, pharmacokinetic and safety analyses.
Patient baseline characteristics are presented in Table 1. The mean duration of hypertension was 9.2 years and 15.0% patients had concomitant diabetes mellitus and 42.5% had hyperlipidemia. Concomitant renal disease was diagnosed in 30 patients (75.0%), with chronic glomerulonephritis being the most common occurring disorder in 19 patients (47.5%), followed by diabetic nephropathy in four patients (10.0%). Prior antihypertensive medication was used by 30 patients (75%), with dihydropyridine derivatives (21 patients), Ang II antagonists (20 patients) and ACEIs (6 patients) being the most common ones. During the study, six patients (15%) used non-potassium-sparing concomitant diuretics (with no change in drug or dosing regimen), the most common being furosemide by four patients and indapamide by two patients.
Efficacy
The antihypertensive effect of aliskiren on msDBP was evident during the first 2 weeks of treatment, msDBP (±s.d.) fell from 99.3±3.7 mm Hg at baseline to 90.3±8.8 mm Hg at Week 2, followed by a further decrease to 88.3±10.2 mm Hg at Week 4 and 87.8±9.8 mm Hg at Week 6. After Week 6, msDBP was stable at 87.4±10.0 mm Hg until Week 8. Mean (±s.d.) decrease in msDBP from baseline to Week 8 endpoint was 11.6±9.7 mm Hg (Figure 3). At Week 2, msSBP (±s.d.) fell from 163.3±11.7 mm Hg at baseline to 153.6±15.1 mm Hg, followed by a further decrease to 149.5±15.2 mm Hg at Week 4. After Week 4, msSBP remained stable at Week 6 (148.9±16.6 mm Hg) and at Week 8 (149.1±18.4 mm Hg). The mean (±s.d.) decrease in msSBP from baseline to Week 8 endpoint was 13.9±16.6 mm Hg (Figure 3).
Overall, 65.0% of patients had an msSBP or msDBP response at the Week 8 endpoint. The overall blood pressure control rate at each assessment increased over time and was 25.0% at Week 2, 25.6% at Week 4, 27% at Week 6 and 30% at the Week 8 endpoint. The majority of the patients (67.5%) in this study were on 300 mg of aliskiren at Week 8.
Pharmacokinetics
A dose-related increase in trough plasma aliskiren levels was observed, with trough levels reaching steady state 2–4 weeks after initiating aliskiren or increasing the dose. Mean (±s.d.) trough plasma aliskiren levels at Week 8 were 5.3±2.8 for 75 mg, 20.3±11.9 for 150 mg and 34.8±23.6 ng ml–1 for 300 mg.
Safety and tolerability
Aliskiren was well tolerated in this study (Table 2). Adverse events were reported for 52.5% patients and 15.0% patients had adverse events that were suspected of being related to aliskiren. The most frequently reported adverse events were nasopharyngitis, back pain and dizziness (each in 5.0% patients). Most of the adverse events were mild-to-moderate in severity. Three patients (7.5%) discontinued the study because of the adverse events of increased blood pressure (n=1), cerebral infarction (n=1) and proteinuria (n=1).
Serious adverse events were reported in two patients (5.0%) and included cerebral infarction, which also led to discontinuation as stated above in one patient, and urinary retention and cystitis (cystitis reported after completion of study) in the other patient. A possible relation to the study drug could not be excluded for the cerebral infarction. No death was reported during the study.
The mean laboratory values for serum creatinine, CCr, and urinary protein/creatinine ratio from baseline to Week 8 endpoint were essentially unchanged with no apparent worsening. An elevated blood urea nitrogen (>50% increase from baseline) was observed in four patients and an elevated urinary protein/creatinine ratio (two-fold increase from baseline) was observed in two patients. For one of the patients with increased protein creatinine ratio, serum creatinine and blood pressure tended to higher levels from the start of the study. For the other patient, there were no significant changes in serum creatinine or CCr; however, the increase in urinary protein/creatinine ratio could have been due to a failure to control blood pressure or washout of prior therapeutic drugs (amlodipine besilate, perindopril erbumine, valsartan and dipyridamole). There were no reports of adverse events related to renal function. Serum potassium levels increased by ⩾20% in four out of six patients whose baseline CCr was <30 ml min–1.
Increases in serum potassium were observed in six patients; none of these changes were reported as adverse events. Serum potassium levels of >5.5 and <6 mEq l–1 were observed in three patients at some point during treatment, and returned to reference range at the endpoint. Three patients had serum potassium levels ⩾6 mEq l–1, of which one patient tended to ⩾6 mEq l–1 levels from the start of treatment (did not exhibit any related clinical symptoms), another had a history of hyperkalemia and was on calcium polystyrene and for the third, the value returned to reference range.
Four patients met the criteria for a decrease in orthostatic blood pressure at some point from baseline to Week 8 endpoint; all were asymptomatic. Throughout the treatment period, no changes were observed in pulse rate, body mass index and body weight. One patient exhibited an ECG abnormality (supraventricular extrasystole) at Week 8 that was suspected to be study-drug related, was mild in severity and required no treatment.
Discussion
This was the first study to evaluate the efficacy, safety and tolerability of aliskiren in Japanese hypertensive patients with renal dysfunction. The antihypertensive effect of aliskiren (mean decrease from baseline to Week 8 endpoint of 11.6 mm Hg for msDBP and 13.9 mm Hg for msSBP) in this population was similar to that observed in Japanese hypertensive patients without renal dysfunction. In an earlier study by Kushiro et al.,12 treatment with aliskiren for 8 weeks at doses ranging from 75 to 300 mg once daily resulted in mean decrease of 7.22–10.72 mm Hg for msDBP and 8.57–14.09 mm Hg for msSBP. Blood pressure reductions with aliskiren in this study were evident within 2 weeks of the first dose, consistent with earlier observations in other studies that evaluated the efficacy of aliskiren in Japanese and Caucasian hypertensive patients without renal dysfunction.10, 12, 14
In this study, a high proportion of patients (65%) achieved a treatment response by Week 8 endpoint (absolute msSBP <140 mm Hg or a decrease of ⩾20 mm Hg from baseline; or absolute msDBP <90 mm Hg or a decrease of ⩾10 mm Hg from baseline). Achieving target blood pressure is of utmost importance for improving renal outcomes, as reduction in blood pressure is known to exert a nephroprotective effect.3, 15, 16
In these patients with renal dysfunction, mean trough plasma aliskiren concentrations increased with dose and reached steady state 2–4 weeks after the start of dosing or up-titration of the dose. The results from an earlier study assessing the effects of renal impairment on pharmacokinetics of aliskiren (300 mg once daily) in patients with mild-to-moderate renal dysfunction and healthy volunteers showed that although exposure is increased by renal impairment, the increase in exposure does not correlate with the severity of renal impairment.17 Moreover, healthy volunteer studies have shown that renal excretion has a minor role in aliskiren elimination, with only 0.1–1.0% of an oral dose being excreted in urine.8 As renal clearance of aliskiren represents a small fraction of total clearance, adjustment of the initial dose is unlikely to be required in patients with moderate renal impairment. Therefore, aliskiren may be a useful alternative to other agents such as the majority of ACEIs, which require dose adjustment for patients with renal dysfunction.18
In this study, the incidence of adverse events was low and the adverse event profile was similar to that observed in earlier studies in hypertensive patients with and without renal dysfunction.8, 12, 17 There were no reports of adverse events related to renal function. Changes in serum creatinine, CCr, and the urinary protein/creatinine ratio were minimal during the study and none of these changes resulted in any adverse event related to the renal function.
In patients with renal dysfunction, inhibition of RAAS with ACEIs, ARBs or both often induce functional increases in serum creatinine levels in an early stage of the treatment.19 This is generally attributed to decreased glomerular capillary pressure because of preferential vasodilation of efferent arteriole as compared with afferent arterioles. In this study, we did not observe such increases in serum creatinine. This observation of unchanged serum creatinine is consistent with earlier studies that used aliskiren.20, 21 The mechanism for the difference between aliskiren and either ACEIs or ARBs is not clear from this study. However, it may be related to unique features of aliskiren, namely strong accumulation in the renal tissue and very powerful renal vasodilatory action.22 It has been shown that in human beings, renal blood flow increased more with aliskiren than with either ARBs or ACEIs.23 As renal blood flow is a powerful determinant of glomerular filtration rate (GFR), administration of aliskiren may have induced little change in GFR even in the presence of decreased glomerular capillary pressure because of decreased Ang II. However, clarification of the significance of these findings awaits further investigation.
In this study, transient increases in serum potassium levels were observed in five patients; none of these changes were reported as adverse events. Transient elevations in serum potassium levels (>5.5 mmol l–1) have been earlier observed with aliskiren treatment in hypertensive patients.24, 25 Mild increases in potassium levels were expected with agents that block the RAAS, as observed with ACEIs and ARBs, because of changes in both GFR and aldosterone secretion.26, 27 Therefore, it is recommended that serum potassium levels be monitored during aliskiren treatment in patients with severe renal dysfunction.
Beyond its antihypertensive effect, recent data suggest that aliskiren may have a beneficial effect on kidney function by slowing the decline in GFR and reducing the urinary albumin/creatinine ratio. In the AVOID study of hypertensive patients with type 2 diabetes and nephropathy, patients treated with aliskiren plus losartan combination therapy for 6 months had a smaller decline in GFR than patients treated with placebo plus losartan, even though blood pressure was similar and well controlled in both treatment groups.21 In the aliskiren plus losartan group, 25% patients experienced a reduction in the urinary albumin/creatinine ratio of ⩾50% compared with 12.5% patients in the placebo plus losartan group. Additional research has shown that RAAS blockade with direct renin inhibition can decrease albuminuria. In a study by Persson et al.,28 aliskiren 300 mg once daily for 28 days was associated with a 44% decrease in the urinary albumin/creatinine ratio and a reduction in 24-h systolic blood pressure (after 7 days) in patients with type 2 diabetes and albuminuria. In this study, the urinary protein/creatinine ratio was essentially unchanged, which could be a consequence of the short treatment period of 8 weeks and majority (85%) of the patients being non-diabetic. Moreover, this study was designed only to assess the safety and tolerability of aliskiren in these hypertensive patients with renal dysfunction, and not its renoprotective effect.
In conclusion, results from this study show that treatment with aliskiren is effective and well tolerated in Japanese patients with mild-to-moderate hypertension and renal dysfunction. Monitoring of renal function and electrolytes during aliskiren therapy should be considered in patients with moderate or severe renal dysfunction, particularly in those with a history of hyperkalemia.
References
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J . Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365: 217–223.
Wong ND, Lopez VA, L'Italien GL, Chen R, Kline S, Franklin S . Inadequate control of hypertension in US adults with cardiovascular disease co morbidities in 2003–2004. Arch Int Med 2007; 167: 2431–2436.
Salvetti A, Mattei P, Sudana I . Renal protection and antihypertensive drugs. Drugs 1999; 57: 665–693.
Ferrario C . Role of angiotensin II in cardiovascular disease—therapeutic implications of more than a century of research. J Renin Angiotensin Aldosterone Syst 2006; 7: 3–14.
Chobanian A, Bakris G, Black HR, Cushman WC, Green LA, Izzo Jr JL, Jones DW, Materson BJ, Oparil S, Wright Jr JT, Roccella EJ, the National High Blood Pressure Education Program Coordinating Committee. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003; 289: 2560–2572.
Jensen C, Herold P, Brunner H . Aliskiren: the first renin inhibitor for clinical treatment. Nature Rev 2008; 7: 399–410.
Azizi M, Menard J . Combined blockade of the renin–angiotensin system with angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists. Circulation 2004; 109: 2492–2499.
Nussberger J, Wuerzner G, Jensen C, Brunner HR . Angiotensin II suppression in humans by the orally active renin inhibitor aliskiren (SPP100): comparison with enalapril. Hypertension 2002; 39: E1–E8.
Gradman AH, Schmieder RE, Lins RL, Nussberger J, Chiang Y, Bedigian MP . Aliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patients. Circulation 2005; 111: 1012–1018.
Stanton A, Jensen C, Nussberger J, O'Brien E . Blood pressure lowering in essential hypertension with an oral renin inhibitor, aliskiren. Hypertension 2003; 42: 1137–1143.
Mori H, Ukai H, Yamamoto H, Saitou S, Hirao K, Yamauchi M, Umemure S . Current status of antihypertensive prescription and associated blood pressure control in Japan. Hypertens Res 2006; 29: 143–151.
Kushiro T, Itakura H, Abo Y, Gotou H, Terao S, Keefe DL . Aliskiren, a novel oral renin inhibitor, provides dose-dependent efficacy and placebo-like tolerability in Japanese patients with hypertension. Hypertens Res 2006; 29: 997–1005.
Japanese Society of Hypertension. Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2004). Hypertens Res 2006; 29: S1–S106.
Oh B, Mitchell J, Herron J, Chung J, Khan M, Keefe DL . Aliskiren, an oral rennin inhibitor provides dose-dependent efficacy and sustained 24-h blood pressure control in patients with hypertension. J Am Coll Cardiol 2007; 49: 1157–1163.
Susic D, Frohlich E . Nephroprotective effect of antihypertensive drugs in essential hypertension. J Hypertens 1998; 16: 555–567.
Abbott K, Basta E, Bakris L . Blood pressure control and nephroprotection in diabetes. J Clin Pharmacol 2004; 44: 431–438.
Vaidyanathan S, Bigler H, Yeh C, Bizot M, Dieterich H, Howard D, Dole W . Pharmacokinetics of the oral direct renin inhibitor aliskiren alone and in combination with irbesartan in renal impairment. Clin Pharmacokinet 2007; 46: 661–675.
Hoyer J, Schulte KL, Lenz J . Clinical pharmacokinetics of angiotensin converting enzyme inhibitors in renal failure. Clin Pharmacokinet 1993; 24: 230–254.
Kean WF, Eknoyan G . Proteinurai, albuminuria, risk, assessment, detection (PARADE): positional paper of the National Kidney Foundation. Am J Kidney Dis 1999; 33: 1004–1010.
McMurray JV, Pitt B, Latini R, Maggioni AP, Solomon SD, Keefe DL, Ford J, Verma A, Lewsey J . Effects of the oral direct renin inhibitor aliskiren in patients with symptomatic heart failure. Circ Heart Fail 2008; 1: 17–24.
Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK . Aliskiren combined with losartan in type 2 diabetes and nephropathy. NEJM 2008; 358: 2433–2446.
Feldman DL, Jin L, Xuan H, Contrepas A, Zhou Y, Webb RL, Mueller DM, Feldt S, Cumin F, Maniara W, Persohn E, Schuetz H, Jan Danser AH, Nguyen G . Effects of aliskiren on blood pressure, albuminuria, and (pro)renin receptor expression in diabetic TG(mRen-2)27 Rats. Hypertension 2008; 52: 130–136.
Fisher NDL, Jan Danser AH, Nussberger J, Dole WP, Hollenberg NK . Renal and hormonal responses to direct renin inhibition with aliskiren in healthy humans. Circulation 2008; 117: 3199–3205.
Kushiro T, Itakura H, Abo Y, Gotou H, Terao S, Keefe DL . Long-term safety and efficacy of aliskiren, an oral direct renin inhibitor, in japanese patients with hypertension. J Clin Hypertens 2007; 9 (5 suppl A): A169. Abstract P-406.
Andersen K, Weinberger MH, Egan B, Constance CM, Ali MA, Jin J, Keefe DL . Comparative efficacy and safety of aliskiren, an oral direct rennin inhibitor, and rampipril in hypertension: a 6-month, randomized, double-blind trial. J Hypertens 2008; 26: 589–599.
Textor SC, Bravo EL, Fouad FM, Tarazi RC . Hyperkalemia in azotemic patients during angiotensin converting enzyme inhibition and aldosterone reduction with captopril. Am J Med 1982; 73: 719–725.
Kostis JB, Shelton B, Gosselin G, Goulet C, Hood Jr WB, Kohn RM, Kubo SH, Schron E, Weiss MB, Willis PW, Young JB, Probstfield J . Adverse effects of enalapril in the studies of left ventricular dysfunction (SOLVD). SOLVD Investigators. Am Heart J 1996; 131: 350–355.
Persson F, Rossing P, Schjoedt KJ, Juhl T, Tarnow L, Stehouwer CD, Schalkwijk C, Boomsma F, Frandsen E, Parving HH . Time course of the antiproteinuric and antihypertensive effects of direct renin inhibition in type 2 diabetes. Kidney Int 2008; 73: 1419–1425.
Acknowledgements
This study was supported by Novartis Pharma AG (Basel, Switzerland). We acknowledge the investigators and other staff members who participated in this study. The authors were assisted in the preparation of this manuscript by Sunita Cheruku and Ashish Agarwal from Medical & Scientific Clinical Documentation Division, Novartis, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ito, S., Nakura, N., Le Breton, S. et al. Efficacy and safety of aliskiren in Japanese hypertensive patients with renal dysfunction. Hypertens Res 33, 62–66 (2010). https://doi.org/10.1038/hr.2009.175
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2009.175
Keywords
This article is cited by
-
Efficacy analysis of the renoprotective effects of aliskiren in hypertensive patients with chronic kidney disease
Heart and Vessels (2013)
-
Additive renoprotective effects of aliskiren on angiotensin receptor blocker and calcium channel blocker treatments for type 2 diabetic patients with albuminuria
Hypertension Research (2012)
-
Aliskiren-associated acute kidney injury in a patient with pre-existing chronic kidney disease and dilated cardiomyopathy
Clinical and Experimental Nephrology (2012)
-
Beneficial effect of aliskiren combined with olmesartan in reducing urinary protein excretion in patients with chronic kidney disease
International Urology and Nephrology (2012)
-
Effects of Aliskiren on blood pressure and the predictive biomarkers for cardiovascular disease in hemodialysis-dependent chronic kidney disease patients with hypertension
Hypertension Research (2011)