Abstract
Vitis species, including grapevine, produce a class of secondary metabolites called stilbenes that are important for plant disease resistance and can have positive effects on human health. Mitogen-activated protein kinase (MAPK) signaling cascades not only play key roles in plant defense responses but also contribute to stilbene biosynthesis in grapevine. MAPKKKs function at the upper level of the MAPK network and initiate signaling through this pathway. In this study, a Raf-like MAPKKK gene, VqMAPKKK38, was identified and functionally characterized from the Chinese wild grapevine V. quinquangularis accession ‘Danfeng-2’. We observed that VqMAPKKK38 transcript levels were elevated by powdery mildew infection, high salinity conditions and chilling stresses, as well as in response to treatments by the hormones salicylic acid (SA), methyl jasmonate (MeJA), ethylene (Eth) and abscisic acid (ABA). In addition, based on both transient overexpression and gene suppression of VqMAPKKK38 in grapevine leaves, we found that VqMAPKKK38 positively regulates stilbene synthase transcription and stilbene accumulation probably by mediating the activation of the transcription factor MYB14. In addition, both hydrogen peroxide (H2O2) and calcium influx activated VqMAPKKK38 expression and stilbene biosynthesis, which suggests that VqMAPKKK38 may be involved in the calcium signaling and ROS signaling pathways.
Similar content being viewed by others
Introduction
As sessile organisms, plants are constantly exposed to a wide range of biotic and abiotic stresses and have evolved a large number of sophisticated signal transduction mechanisms to both regulate their development and enhance their resistance to these stressors. For example, the mitogen-activated protein kinase (MAPK) cascade is commonly used by eukaryotes to transduce extracellular stimuli into intracellular responses.1 The basic components of an MAPK cascade comprise three interconnected kinase modules: MAPKKK/MEKK, MAPKK/MKK and MAPK/MPK.2 MAPKKK proteins function in the beginning of the cascade, receiving signals from upstream sensors to initiate the pathway, and activate the MAPKK proteins by phosphorylating the serine/threonine residues in a conserved motif (Ser/Thr-X3-5-Ser/Thr, X indicating any amino acid) of the activation loop.3,4 The activated MAPKK proteins in turn mediate the activation of downstream MAPK proteins through the phosphorylation of threonine and/or tyrosine residues in the T-X-Y motif.3,4 The phosphorylated MAPK proteins then act as regulators of multiple effector proteins in the nucleus or cytoplasm that can be transcription factors, cytoskeletal components and protein kinases.3,4 MAPK cascades thus connect upstream signals to downstream targets and participate in the adaptation to a broad range of pathogenic and environmental threats such as bacterial or fungal attack, viral infection, wounding, high salinity, drought, osmotic stress, ultraviolet (UV) irradiation and temperature extremes.5
Several three-kinase modules have been functionally characterized in plants. Recent studies in Arabidopsis thaliana showed that the MAPKKK17/18-MKK3-MPK1/2/7/14 cascade operates downstream from abscisic acid (ABA)-induced stress signaling6 and plays an important role in ABA-modulated leaf senescence.7 The AIK1-MKK5-MPK6 module was shown to be activated by ABA and to regulate ABA responses, including root development and stomatal behavior.8 Furthermore, two MAPK cascades, MEKK1-MKK1/2-MPK4 and MEKK1-MKK4/5-MPK3/6, have been shown to play a role in flagellin-induced signal transmission,9 while the CTR1-MKK9-MPK3/6 cascade is involved in ethylene-regulated signaling.10 In addition, in tobacco, the two modules, MAPKKKα-MEK2-SIPK and NPK1-MEK1/NQK1-NTF6/NRK1, contribute to pathogen defense and plant cytokinesis,11–13 and tomato (Solanum lycopersicum) MAPKKKα-MKK2/MKK4-MPK2/MPK3 is a component of the Pto-mediated effector triggered immunity (ETI) pathway.14
Whole-genome sequencing of numerous plant species has generated a valuable resource in the form of an inventory of MAPK families from those species. For instance, there are 20 MAPKs, 10 MAPKKs and at least 80 MAPKKKs in A. thaliana, while the grapevine (Vitis vinifera) genome contains 14 MAPKs, five MAPKKs and 62 MAPKKKs.10,15–17 The MAPKKKs of a given species show enhanced sequence diversity compared to members of the MAPKK or MAPK families.18 On the basis of the sequence in the conserved kinase domain, the MAPKKK family in higher plants has been classified into three clades: the MEKK-like subfamily has a conserved signature (G(T/S)Px(W/Y/F)MAPEV); the ZIK subfamily contains a GTPEFMAPE(L/V)Y sequence, and the Raf-like subfamily has a specific GTxx(W/Y)MAPE signature.18,19 MEKK1 is the most thoroughly studied MAPKKK in A. thaliana and is known to be involved in multiple stress responses, including flagellin signaling,9 wounding,20 and cold and salt stimuli.21 Two well-known Raf-like MAPKKKs, CTR1 (Constitutive Triple Response 1) and EDR1 (Enhanced Disease Resistance 1), function as negative regulators of the ethylene 22 and pathogen defense responses,23 respectively. In cotton (Gossypium hirsutum), GhRaf19 has been shown to enhance the tolerance to chilling stress but to decrease drought and salt stress resistance,24 while GhMKK5 is significantly triggered by salicylic acid (SA) and induces the transcription of pathogenesis-related (PR) genes.25 Recently, A. thaliana AIK1 and MAPKKK17/18 were identified as key regulators of ABA signal transduction,7,8 but relatively little information is available regarding MAPKKKs in grapevine. A genome-wide analysis of MAPK cascades in V. vinifera revealed 21 MEKKs, 12 ZIKs and 29 Rafs among the 62 MAPKKKs.16 Expression profiles of 45 grapevine MAPKKK genes following exposure to various stress conditions suggested that these candidate MAPKKK genes may participate in responses to powdery mildew, drought, SA, ethylene (Eth) and hydrogen peroxide (H2O2).26 However, a more detailed characterization of individual MAPKKKs is required to better define their biological and physiological roles.
China is a major biodiversity center for Vitis,27 and Chinese wild grapevines provide valuable gene pools with a number of resistance factors that are thought to be important for pathogen immunity. The Chinese wild grapevine species V. quinquangularis, particularly the accession ‘Danfeng-2’, has attracted attention because of its high level of resistance to pathogen infection and its high content of stilbene-type phytoalexins.28–30 Stilbenes are secondary metabolites that help promote resistance to a diverse range of pathogens, and they also have pharmacological value.31–33 The accumulation of stilbenes can be induced by factors such as pathogen infection,32 ozone damage,34 wounding,35 salt stress36 and UV irradiation.34,37 Perhaps the most widely studied stilbene, resveratrol, is synthesized by a side branch of the well-characterized phenylalanine/polymalonate pathway where the final step is catalyzed by stilbene synthase (STS).38 The R2R3-MYB-type transcription factors MYB14 and MYB15 are thought to regulate the biosynthesis of stilbenes by up-regulating STS transcription.39 It has also been suggested that MAPK cascades are required for stilbene biosynthesis, and a specific MAPK cascade inhibitor (PD98059) can efficiently suppress the activation of STS by flagellin 22 (flg22), a bacterial elicitor harpin,40 or by SA.41 PD98059 can also block the induction of MYB14 by flg22 in grapevine cell suspension cultures.42
In this study, we sought to identify genes in the MAPK pathway that are involved in the regulation of stilbene accumulation in V. quinquangularis. We focused on VqMAPKKK38, a Raf-like subfamily member that is responsive to various stressors and examined its role in signal transduction and stilbene biosynthesis. We present functional studies involving transient overexpression and suppression using RNAi in grapevine leaves and propose a mechanism by which VqMAPKKK38 promotes stilbene biosynthesis, as well as how its involvement in signaling is triggered by calcium and reactive oxygen species (ROS).
Materials and methods
Plant materials and stress treatments
Chinese wild V. quinquangularis accession Danfeng-2 was cultivated in the grape germplasm resources garden at the Northwest A&F University, Yangling, Shaanxi, China. Samples of young leaves (the second to fourth leaf from the tip), mature leaves (dark-green leaves collected when berries were enlarging), stems (the woody stem), inflorescences (with single flowers in dense groups), young berries (berries were enlarging, 25 days after anthesis) and mature berries (berries were harvest-ripe, 80 days after anthesis) were collected for expression analyses.
Fresh young leaves were collected and subjected to different forms of stress. Powdery mildew (Erysiphe necator) inoculation was carried out as described previously,43 and inoculated leaves were collected at 0, 12, 24, 48, 72, 96 and 120 h post-inoculation. For abiotic stress treatments, young leaves were either wounded with sterile scissors, sprayed with aqueous 250 mM NaCl, or exposed to low (4 °C) or high temperature (37 °C) for 0, 0.5, 1, 2, 6 or 10 h. Treatment with the signaling molecules was carried out by spraying the young leaves with one of the following solutions: 100 μM SA, 100 μM MeJA, 100 μM Eth, 100 μM ABA, 5 mM CaCl2 or 1% H2O2 (w/v). The leaves were harvested at 0, 0.5, 1, 2, 6 and 10 h post-treatment. The leaves were pretreated with 20 μM GdCl3 for 30 min before 5 mM CaCl2 was administered to study the roles of Ca2+ and ROS. Dimethylthiourea (DMTU), an H2O2 scavenger, was used to pretreat the leaves for 30 min before 1% H2O2 was administered. GdCl3 or DMTU was also added without a subsequent treatment to assess the effect of the inhibitors on the leaves. Leaves treated with the solvent in which the elicitors were dissolved served as negative controls. Three samples were treated in each treatment, and each treatment was repeated three times.
Expression analysis
Total RNA was extracted from leaves with the EZNA Total RNA kit II (Omega Bio-Tek) and reverse-transcribed into complementary DNA (cDNA) using Prime Script Reverse Transcriptase (TaKaRa) following the manufacturer’s instructions. Semi-quantitative reverse transcription-PCR was performed with the following parameters: 94 °C for 3 min followed by 30 cycles of 94 °C for 30 s, 58 °C for 30 s and 72 °C for 1 min. The amplified products were separated on a 1% agarose gel and visualized with ethidium bromide. Quantitative real-time PCR (qRT-PCR) was carried out as described previously.44 The specific primers used for gene expression analysis are shown in Supplementary Table S1. Gene transcript levels were quantified with normalization to grapevine GAPDH (GenBank accession no. GR883080) and EF1γ (GenBank accession no. AF176496) as internal standards. Each experiment was carried out with three biological replicates, and each biological sample was analyzed in three technical replicates.
Cloning and VqMAPKKK38 sequence analysis
The specific primers used to isolate the full-length VqMAPKKK38 cDNA (see Supplementary Table S1) were designed according to the homologous sequences from the reference genome of V. vinifera cv. ‘Pinot Noir’ clone P40024.45 The VqMAPKKK38 gene is located on the fifth chromosome according to a BLAST search in the Genoscope Genome Browser (http://www.genoscope.cns.fr/blat-server/cgi-bin/vitis/webBlat). DNAMAN software was used to carry out amino-acid sequence alignment analyses of four Raf proteins, including VqMAPKKK38, VviRaf23 (Gene ID: VIT05s0094g01080), AtRaf23 (Gene ID: At2G31800) and BnaRaf23 (GenBank: AHL77715.1). The phylogenetic tree was constructed using MEGA 7 software to analyze the evolutionary relationship between these proteins.
Plasmid construction and transient expression assays in grapevine
To generate the over-expression construct, the amplified product (open reading frame of VqMAPKKK38) was inserted into the pART-CAM-S vector46 after digestion with SacI and ClaI. To create the silencing construct, a fragment from 545 to 1405 bp of VqMAPKKK38 was isolated as sense and antisense sequences. Both sense and antisense sequences were cloned into pKANNIBAL,47 and then into pART27 48 after restriction digestion by NotI. Each sequenced plasmid was transformed separately into Agrobacterium tumefaciens strain GV3101 using electroporation and then introduced into 8-week-old V. quinquangularis leaves by Agrobacterium-mediated transient expression as described previously.44
Stilbene quantification
Stilbene levels in the transgenic leaves were measured as described previously44 with minor modifications. The leaf samples were ground in liquid nitrogen, homogenized and extracted with 80% methanol, and then 20 μl of each sample was quantified by high pressure liquid chromatography (Shimadzu Corp, Kyoto, Japan) with a detection wavelength of 306 nm. The mobile phase was 0.5% (v/v) formic acid and acetonitrile (ACN). The mobile phase elution procedure was carried out as follows: 0–8 min, 10–18% ACN; 8–10 min, 18% ACN; 10–15 min, 18–25% ACN; 15–18 min, 25–35% ACN; 18–25 min, 35% ACN; 25–30 min, 35–70% ACN. The sample peaks and standard chemical peaks were calculated using trans-resveratrol and trans-piceid (Sigma-Aldrich Inc., http://www.sigmaaldrich.com/) as external standards. We obtained the cis-piceid standard by the photoisomerization of trans-piceid under UV irradiation. The 50% ethanol solution containing 400 μM of trans-piceid was irradiated at 366 nm for 3 h, and the resulting cis-piceid standard was stored in total darkness.37
Results
VqMAPKKK38 cloning and sequence analysis
The open reading frame (ORF) of VqMAPKKK38 was amplified by PCR with specific primers using the cDNA derived from leaves of V. quinquangularis accession Danfeng-2. The PCR-amplified fragment was 1,419 bp in length and predicted to encode a 472-amino-acid protein with a catalytic kinase domain from residue Gln203 to Leu450 and a Ser/Thr kinase active site (VIHCDLKPKNILL; Figures 1a and b). A DNA sequence alignment with VviMAPKKK38 from V. vinifera (VviRaf23, ID:VIT05s0094g01080) revealed 100% identity, so the fragment was named VqMAPKKK38 and considered to be a member of the Raf-like subfamily. A phylogenetic analysis with other MAPKKK proteins and alignment with other Raf-like protein sequences further supported the classification of VqMAPKKK38 (Figures 1b and c).
Chromosome location (a), sequence alignment analysis (b) and phylogenetic analysis (c) of VqMAPKKK38. The Raf proteins used for the alignment were VviRaf23 (Gene ID: VIT05s0094g01080), AtRaf23 (Gene ID: At2G31800), and BnaRaf23 (GenBank: AHL77715.1). Identical residues are shaded gray. The Raf motif is marked with triangles. The active site (VIHCDLKPKNILL) is boxed, and the kinase catalytic domain is located between the two arrows. The phylogenetic analysis of VqMAPKKK38 and MAPKKK proteins from Vitis vinifera, Arabidopsis thaliana, Brassica napus and Gossypium hirsutum was carried out using MEGA 7 software.
VqMAPKKK38 expression in different organs and in response to environmental stimuli and hormone treatments
qRT-PCR revealed that VqMAPKKK38 transcripts were expressed in all of the organs tested, including young leaves, mature leaves, stems, inflorescences, young berries and mature berries. Since the highest levels were observed in young leaves (Figure 2a), these were used for subsequent expression studies.
VqMAPKKK38 expression analysis in various organs and in response to a range of stresses and hormone treatments. (a) Expression analysis of VqMAPKKK38 in different organs determined by qRT-PCR. (b) Time course experiment determining VqMAPKKK38 transcript levels in response to inoculation with powdery mildew, treatment with 250 mM NaCl, chilling, heat and wounding (c–f), as well as to treatments with 100 μM salicylic acid (SA), methyl jasmonate (MeJA), ethylene and abscisic acid (ABA) (g–j). Grapevine GAPDH and EF1γ were used as internal standards. The results are indicated with mean values and s.e. from three biological replicates. Different letters represent significant differences (P<0.05) determined by one-way analysis of variance (ANOVA) and post hoc comparison test (Student–Newman–Keuls) using SPSS 21.0 for Windows (SPSS Inc., Chicago, IL, USA).
It has been established that MAPK cascades function in basal immunity 49 and are activated in response to various abiotic and biotic stresses.5 Therefore, we analyzed the expression profiles of VqMAPKKK38 during powdery mildew infection, as well as following salt, chilling, heat and wounding treatments. After inoculation with grapevine powdery mildew (Uncinula necator), the expression of VqMAPKKK38 remained at basal levels until 72-hour post inoculation (hpi), and then increased at 96 and 120 hpi. (Figure 2b). Following both salt and chilling treatments, the abundance of the VqMAPKKK38 transcripts was significantly elevated at 0.5 h, and then remained at high levels throughout the experiment (Figures 2c and d). In contrast, neither the heat or the wounding treatments stimulated VqMAPKKK38 gene expression for the 10 h duration of the experiments (Figures 2e and f).
Hormones play central roles in plant growth and stress responses, and a number of hormone signals are known to be active via MAPK cascades in plant cells.50 We examined VqMAPKKK38 expression in response to phytohormones and found that SA induced expression within 30 min of treatment, and this persisted over the entire 10 h duration of the experiment (Figure 2g). After MeJA application, the VqMAPKKK38 transcript levels were strongly up-regulated after 1 h and peaked at 6 h, followed by a sharp decline at 10 h (Figure 2h). Treatment with Eth or ABA led to an upregulation of VqMAPKKK38 expression at 6 and 2 h, respectively (Figures 2i and j).
VqMAPKKK38 promotes stilbene biosynthesis in grapevine
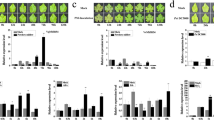
Our previous studies of grapevine suspension culture cells showed that MAPK signaling is necessary for the activation of STS promoters by SA.41 As shown in Figure 2, VqMAPKKK38 expression was strongly induced by SA, so we investigated whether VqMAPKKK38 plays a role in SA-triggered stilbene biosynthesis. An Agrobacterium-mediated transient expression system was used to overexpress (OE) or suppress via RNA interference (RNAi) VqMAPKKK38 expression in grapevine leaves. pART-CAM-S and pART27 without a target gene served as negative controls. Both semi-quantitative reverse transcription-PCR and qRT-PCR analysis confirmed that VqMAPKKK38 was successfully overexpressed or silenced (Figure 3a). We measured the stilbene levels in the transformed grapevine leaves before and after 100 μM SA treatment by high-performance liquid chromatography (HPLC). As indicated in Figure 3b, both trans-resveratrol and trans-piceid levels increased after VqMAPKKK38 was overexpressed. In addition, after SA treatment, the VqMAPKKK38 overexpressor accumulated significantly higher levels of these compounds than the control. This suggested that VqMAPKKK38 promotes stilbene accumulation in grapevine. A transient silencing assay further confirmed this hypothesis, since RNAi-VqMAPKKK38 leaves exposed to the SA treatment contained much less trans-resveratrol and trans-piceid than the control leaves. Glucoside cis-piceid was also detected in all overexpressing/silenced leaves but showed the same levels as those observed in the controls.
Stilbene accumulation regulated by VqMAPKKK38. (a) Semi-quantitative RT-PCR (representative agarose gel) and qRT-PCR analysis of VqMAPKKK38 in transgenic grapevine leaves. C1: grapevine leaves harboring the pART-CAM-S vector without the target gene; C2: grapevine leaves harboring the pART27 vector without the target gene. The housekeeping genes GAPDH and EF1γ were used as internal standards. (b) Amounts of stilbenes in transgenic grapevine leaves after 100 μM salicylic acid (SA) treatment. Mock represents a solvent only treatment. The results represent mean values and s.e. from three independent experiments, and different letters represent significant differences (P<0.05) determined by a two-way analysis of variance (ANOVA) and post hoc comparison test (Student–Newman–Keuls) using SPSS 21.0 for Windows (SPSS Inc., Chicago, IL, USA).
To investigate whether the upregulation of stilbene biosynthesis caused by VqMAPKKK38 overexpression was linked to the activation of STS, the expression of several VqSTS genes was determined. qRT-PCR data showed that the expression of the VqSTS genes was significantly induced in OE-VqMAPKKK38 leaves, especially after SA treatment (Figure 4a). In addition, the induction of the VqSTS genes by SA was markedly suppressed in RNAi-VqMAPKKK38 leaves (Figure 4b). These results are consistent with VqMAPKKK38 up-regulating the expression of VqSTSs.
Analysis of VqSTSs transcript levels during transient overexpression (a) and interference (b) of VqMAPKKK38 in grapevine leaves. Grapevine GAPDH and EF1γ were used as internal standards for the measurement. The results represent mean values and s.e. from three independent experiments. Mean values with different letters represent significant differences (P<0.05) determined by a two-way analysis of variance (ANOVA)and post hoc comparison test (Student–Newman–Keuls) using SPSS 21.0 for Windows (SPSS Inc., Chicago, IL, USA).
It has been shown that the R2R3-MYB-type transcription factors MYB14 and MYB15 are responsible for the regulation of STS in grapevine39 and that the activation of MYB14 by flg22 is dependent on MAPK signaling.42 To investigate whether the accumulation of stilbenes and the activation of STS genes regulated by VqMAPKKK38 correlated with induction of these transcription factors, we examined their transcript levels in the leaves of the OE- and RNAi-VqMAPKKK38 lines. In response to SA treatment, the expression of MYB14 was significantly up-regulated in the OE-VqMAPKKK38 leaves and downregulated in the RNAi-VqMAPKKK38 leaves compared with the control (Figure 5a). In contrast, the induction of MYB15 by SA seemed to be marginal, and we concluded that it was not regulated by VqMAPKKK38 (Figure 5b).
Transcript levels of MYB14/MYB15 during transient overexpression and interference of VqMAPKKK38 in grapevine leaves. qRT-PCR data show the transcript abundance of MYB14 and MYB15 in transgenic grapevine leaves after 100 μM salicylic acid (SA) treatment. Mock represents transgenic grapevine leaves treated with solvent only. Quantification is relative to GAPDH and EF1γ. Mean values and s.e. are from three biological replicates. Different letters represent significant difference (P<0.05) determined by a two-way analysis of variance (ANOVA) and post hoc comparison test (Student–Newman–Keuls) using SPSS 21.0 for Windows (SPSS Inc., Chicago, IL, USA).
VqMAPKKK38 activation by the calcium ionophore and H2O2
The influx of Ca2+, generation of ROS and activation of MAPK cascades are early signaling events associated with immune responses in plants. During unfavorable conditions, these factors are able to trigger and regulate one another,49,51–54 leading us to hypothesize that VqMAPKKK38 expression could be induced by changing the cytoplasmic Ca2+ or ROS levels. Young V. quinquangularis leaves were treated with 5 mM CaCl2 or 1% H2O2 (w/v) and sampled at 0.5, 1, 2, 6 and 10 h post-treatment. qRT-PCR analysis revealed that VqMAPKKK38 expression was significantly induced by exposure to either CaCl2 or H2O2 (Figures 6a and b).
Regulation of VqMAPKKK38 by calcium influx and hydrogen peroxide (H2O2). (a and b) Time course of the expression of VqMAPKKK38 in response to 5 mM CaCl2 and 1% H2O2 (w/v) treatments, respectively. (c) Induction of VqMAPKKK38 was measured after pretreatment of young V. quinquangularis leaves for 30 min with a calcium-influx inhibitor, 20 μM gadolinium chloride (Gd). (d) VqMAPKKK38 expression was measured after pretreatment for 30 min with an H2O2 scavenger, 5 mM dimethylthiourea (DMTU). GAPDH and EF1γ were used as internal standards. The results indicate mean values and s.e. from three biological replicates. Different letters represent significant differences (P<0.05) determined by a one-way analysis of variance (ANOVA) and post hoc comparison test (Student–Newman–Keuls) using SPSS 21.0 for Windows (SPSS Inc., Chicago, IL, USA).
To further investigate the role of Ca2+ and H2O2 in VqMAPKKK38 transcription, we tested the effect of GdCl3, an inhibitor of mechanosensitive calcium channels, on the induction of VqMAPKKK38 by CaCl2, as well as the effect of the H2O2 scavenger dimethylthiourea (DMTU) on the activation of VqMAPKKK38 by H2O2. Compared to the solvent controls, the accumulation of VqMAPKKK38 transcripts significantly increased after adding either CaCl2 or H2O2 for 2 h (Figures 6c and d). When the young leaves were pre-treated with 20 μM GdCl3 for 30 min before the CaCl2 was administered, the VqMAPKKK38 expression was higher than that in the solvent control, but significantly lower than in the group treated with CaCl2 alone (Figure 6c). The pretreatment of leaves with 5 mM DMTU for 30 min significantly decreased the H2O2 induction of VqMAPKKK38 in a manner similar to that of the GdCl3 treatment (Figure 6d). Neither GdCl3 nor DMTU themselves affected the expression of VqMAPKKK38. These results indicated that VqMAPKKK38 acts downstream of both the calcium and ROS signaling, and so either or both might induce VqMAPKKK38 expression.
Stilbene accumulation can be triggered by calcium and H2O2
Since VqMAPKKK38 expression was induced in response to either calcium influx or H2O2 treatment, we examined the potential correlation between stilbene induction and exogenous calcium and H2O2. We found that the accumulation of trans-resveratrol, trans-piceid and cis-piceid markedly increased after treatment with either exogenous CaCl2 or H2O2. In addition, the accumulation of the three types of stilbenes induced by CaCl2 was significantly limited by the calcium channel inhibitor GdCl3, while the H2O2 scavenger DMTU effectively suppressed the H2O2-induced accumulation of trans-resveratrol (Figures 7a and b). These findings suggest that Ca2+ and ROS signaling are involved in stilbene accumulation. qRT-PCR analysis further confirmed that the expression of the STS genes was induced in response to calcium influx and ROS signaling. We found that the induction of VqSTS6, VqSTS19, VqSTS24 and VqSTS32 by the calcium ionophore was strongly inhibited by GdCl3 (Supplementary Figure 1a). Moreover, the activation of VqSTS6, VqSTS19, VqSTS26 and VqSTS32 by H2O2 was suppressed when leaves were pretreated with DMTU before the application of H2O2 (Supplementary Figure 1b).
Regulation of stilbene biosynthesis by calcium influx and hydrogen peroxide (H2O2). Contents of trans-resveratrol, trans-piceid and cis-piceid measured by high pressure liquid chromatography. Mean values and s.e. are from three independent experiments. Different letters represent significant difference (P<0.05) determined by a one-way analysis of variance (ANOVA) and post hoc comparison test (Student–Newman–Keuls) using SPSS 21.0 for Windows (SPSS Inc., Chicago, IL, USA).
Discussion
The resistance to pathogen attack of Chinese wild Vitis species such as V. quinquangularis accession ‘Danfeng-2’, is correlated with high concentrations of trans-resveratrol.28 Our previous study of grapevine cell cultures documented the responsiveness of STS to SA depended on MAPK signaling.41 However, the specific elements of this response pathway were unknown. Recent transcriptome data from four developmental stages of berry material from V. quinquangularis accession ‘Danfeng-2’ were analyzed by our colleagues (SRA; SRP067690), and VqMAPKKK38 was predicted to play a role in the regulation of stilbene accumulation (unpublished). In this study, we investigated the involvement of VqMAPKKK38 in stilbene biosynthesis and signal transduction.
In grapevine, the biosynthesis of resveratrol is catalyzed by the key enzyme STS that is specifically activated by MYB14.39 As previously reported, MAPK signaling can mediate the activation of STS transcription.40,41 Consistent with this, Duan et al.42 confirmed that MAPK cascades are essential in the activation of grapevine MYB14.42 In this study, we show that VqMAPKKK38 overexpression in grapevine leaves can significantly enhance SA-induced stilbene accumulation, accompanied by the strong induction of STS and MYB14 expression. We also observed that the accumulation of stilbenes was almost abolished in RNAi-VqMAPKKK38 transgenic leaves, showing that VqMAPKKK38 is required for stilbene biosynthesis and that a MAPKKK38-based cascade is likely to be involved in this process.
The rapid influx of calcium and the generation of ROS are among the earliest cellular responses to biotic and abiotic stresses.55,56 The levels of Ca2+ and ROS (O2−, H2O2, HO· and NO·) are maintained at low levels in plant cells under normal physiological conditions. However, environmental signals can trigger rapid calcium fluxes and increases in the levels of ROS.57,58 Either of these responses can activate a number of molecular processes, including MAPK signaling.54 In this study, we found that the exposure of young grapevine leaves to either exogenous Ca2+ or H2O2 increased VqMAPKKK38 expression and that this effect was the most pronounced after 1 and 2 h, respectively. Since the influx of Ca2+ and the generation of ROS can directly induce each other,51,52 we used GdCl3 and DMTU to generate additional evidence for the induction of VqMAPKKK38 by calcium and H2O2. Taken together, our results demonstrate that VqMAPKKK38 functions downstream of both the Ca2+ and the ROS signaling pathways and that it responds most rapidly to Ca2+-mediated signaling.
The regulation of stilbene accumulation by signaling events has been widely studied in grapevine suspension cell lines. A number of pathogen elicitors can induce stilbene accumulation, including flg22 and harpin, as can hormones such as SA and JA. This inducibility requires an influx of calcium, an oxidative burst and MAPK cascades.40,41,59,60 The signaling events are often shared among different induction processes, but different elicitors can generate different types of stilbene output, mainly due to the relative sequence of calcium influx and an apoplastic burst.40 Since we found that both calcium and the ROS signaling operate upstream of VqMAPKKK38, we examined the effects of calcium and ROS on STSs transcription and stilbene biosynthesis. The observation that both the expression of STS genes and the accumulation of stilbenes were elevated by Ca2+/H2O2 and could also be inhibited by Ca2+/H2O2 blockers suggested the involvement of Ca2+ and ROS signaling in the regulation of stilbene biosynthesis.
It has been shown that exogenous resveratrol can act as a regulator of the hypersensitive reaction accompanied by a stimulation of an oxidative burst in V. rupestris suspension cells.60 In this study, we found that VqMAPKKK38 is involved in the ROS signaling pathway, raising the possibility that endogenous stilbenes may in turn regulate the upstream acting VqMAPKKK38. We therefore measured the accumulation of VqMAPKKK38 transcripts in VqSTS6-, VqSTS23-, or VqSTS32-overexpressing transgenic grapevine plants both before and after powdery mildew treatment. However, since we observed no difference in the expression of VqMAPKKK38 between transgenic and non-transgenic plants (Supplementary Figure S2), we conclude that there is likely no direct feedback regulation between VqMAPKKK38 and downstream stilbene accumulation in grapevine.
In conclusion, this study provides new insights into the biological roles of a grapevine MAPKKK gene, VqMAPKKK38, that has the same coding sequence as VviMAPKKK38 from V. vinifera (ID: VIT05s0094g01080). qRT-PCR analysis revealed that VviMAPKKK38 expression is strongly induced by Erysiphe necator, SA, ethylene and H2O2,26 which is consistent with our report of the expression of VqMAPKKK38 being induced in response to biotic (E. necator) and abiotic (salt, chilling) stresses, as well as defense-related hormone (SA, MeJA, ABA, Eth) treatments. Since the expression profile may be an indicator of gene function, we hypothesize that VqMAPKKK38 is a stress-inducible gene that is recruited for effective defense against a range of stressors. The evidence from the over- and RNAi-expression experiments with grapevine leaves indicates that VqMAPKKK38 is involved in a stilbene-type phytoalexin biosynthesis by mediating the transcription of STS genes and MYB14. Future studies will focus on identification of the VqMAPKKK38-mediated expression module and on the use of this gene for molecular breeding of grapevine.
References
Dóczi R, Okrész L, Romero AE, Paccanaro A, Bögre L . Exploring the evolutionary path of plant MAPK networks. Trends Plant Sci 2012; 17: 518–525.
Mészáros T, Helfer A, Hatzimasoura E et al. The Arabidopsis MAP kinase kinase MKK1 participates in defence responses to the bacterial elicitor flagellin. Plant J 2006; 48: 485–498.
Mishra NS, Tuteja R, Tuteja N . Signaling through MAP kinase networks in plants. Arch Biochem Biophys 2006; 452: 55–68.
Zhang A, Jiang M, Zhang J, Tan M, Hu X . Mitogen-activated protein kinase is involved in abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants. Plant Physiol 2006; 141: 475–487.
Nakagami H, Pitzschke A, Hirt H . Emerging MAP kinase pathways in plant stress signalling. Trends Plant Sci 2005; 10: 339–346.
Danquah A, Zélicourt A, Boudsocq M et al. Identification and characterization of an ABA‐activated MAP kinase cascade in Arabidopsis thaliana. Plant J 2015; 82: 232–244.
Matsuoka D, Yasufuku T, Furuya T, Nanmori T . An abscisic acid inducible Arabidopsis MAPKKK, MAPKKK18 regulates leaf senescence via its kinase activity. Plant Mol Biol 2015; 87: 565–575.
Li K, Yang F, Zhang G, Song S, Li Y, Miao Y et al. AIK1, a mitogen-activated protein kinase, modulates abscisic acid responses through the MKK5-MPK6 kinase cascade. Plant Physiology 2017; 173: 1391.
Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomezgomez L et al. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 2002; 415: 977–983.
Pitzschke A, Schikora A, Hirt H . MAPK cascade signalling networks in plant defence. Curr Opin Plant Biol 2009; 12: 421–426.
Del PO, Pedley KF, Martin GB . MAPKKKalpha is a positive regulator of cell death associated with both plant immunity and disease. EMBO J 2004; 23: 3072–3082.
Melech-Bonfil S, Sessa G . Tomato MAPKKKε is a positive regulator of cell-death signaling networks associated with plant immunity. Plant J Cell Mol Biol 2010; 64: 379–391.
Takahashi Y, Soyano T, Sasabe M, Machida YA . MAP kinase cascade that controls plant cytokinesis. J Biol Chem 2004; 136: 127–132.
Ekengren SK, Liu Y, Schiff M, Dineshkumar SP, Martin GB . Two MAPK cascades, NPR1, and TGA transcription factors play a role in Pto-mediated disease resistance in tomato. Plant J Cell Mol Biol 2003; 36: 905–917.
Colcombet J, Hirt H . Arabidopsis MAPKs: a complex signalling network involved in multiple biological processes. Biochem J 2008; 413: 217–226.
Çakır B, Kılıçkaya O . Mitogen-activated protein kinase cascades in Vitis vinifera. Front Plant Sci 2015; 6.
Wang G, Lovato A, Liang YH et al. Validation by isolation and expression analyses of the mitogen-activated protein kinase gene family in the grapevine (Vitis vinifera L). Aust J Grape Wine Res 2014; 20: 255–262.
Group M . Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci 2002; 7: 301–308.
Jonak C, Okrész L, Bögre L, Hirt H . Complexity, cross talk and integration of plant MAP kinase signalling. Curr Opin Plant Biol 2002; 5: 415–424.
Yasuda K, Nakamoto T, Yasuhara M, Okada H, Nakajima T, Kanzaki H et al. Activation of AtMEK1, an Arabidopsis mitogen-activated protein kinase kinase, in vitro and in vivo: analysis of active mutants expressed in. Plant J 2002; 29: 637–647.
Teige M, Scheikl E, Eulgem T et al. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol Cell 2004; 15: 141–152.
Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR . CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 1993; 72: 427–441.
Frye CA, Innes RW . An Arabidopsis mutant with enhanced resistance to powdery mildew. Plant Cell 1998; 10: 947–956.
Jia H, Hao L, Guo X, Liu S, Yan Y, Guo X . A Raf-like MAPKKK gene, GhRaf19, negatively regulates tolerance to drought and salt and positively regulates resistance to cold stress by modulating reactive oxygen species in cotton. Plant Sci Int J Exp Plant Biol 2016; 252: 267–281.
Zhang L, Li Y, Lu W, Meng F, Wu C, Guo X . Cotton GhMKK5 affects disease resistance, induces HR-like cell death, and reduces the tolerance to salt and drought stress in transgenic Nicotiana benthamiana. J Exp Bot 2012; 63: 3935.
Wang G, Lovato A, Polverari A et al. Genome-wide identification and analysis of mitogen activated protein kinase kinase kinase gene family in grapevine (Vitis vinifera). BMC Plant Biol 2014; 14: 1–19.
Tröndle D, Schroeder S, Kassemeyer HH, Kiefer C, Koch MA, Nick P . Molecular phylogeny of the genus vitis (vitaceae) based on plastid markers. Am J Bot 2010; 97: 1168–1178.
Shi J, He M, Cao J et al. The comparative analysis of the potential relationship between resveratrol and stilbene synthase gene family in the development stages of grapes (Vitis quinquangularis and Vitis vinifera). Plant Physiol Biochem 2013; 74C: 24–32.
Wan Y, Schwaninger H, He P, Wang Y . Comparison of resistance to powdery mildew and downy mildew in Chinese wild grapes. Vitis 2007; 46: 132–136.
Cheng S, Xie X, Xu Y, Zhang C, Wang X, Zhang J et al. Genetic transformation of a fruit-specific, highly expressed stilbene synthase gene from Chinese wild Vitis quinquangularis. Planta 2016; 243: 1–13.
Adrian M, Jeandet P, Veneau J, Weston LA, Bessis R . Biological activity of resveratrol, a stilbenic compound from grapevines, against botrytis cinerea, the causal agent for gray mold. J Chem Ecol 1997; 23: 1689–1702.
Schnee S, Viret O, Gindro K . Role of stilbenes in the resistance of grapevine to powdery mildew. Physiol Mol Plant Pathol 2008; 72: 128–133.
Roupe KA, Remsberg CM, Yáñez JA, Nm D . Pharmacometrics of stilbenes: seguing towards the clinic. Curr Clin Pharmacol 2006; 1: 81–101.
Gonzálezbarrio R, Beltrán D, Cantos E, Gil MI, Espín JC, Tomásbarberán FA . Comparison of ozone and UV-C treatments on the postharvest stilbenoid monomer, dimer, and trimer induction in var. 'Superior' white table grapes. J Agricult Food Chem 2006; 54: 4222–4228.
Dou D, Tyler BM . Conserved C-terminal motifs required for avirulence and suppression of cell death by Phytophthora sojae effector Avr1b. Plant Cell 2008; 20: 1118–1133.
Ismail A, Riemann M, Nick P . The jasmonate pathway mediates salt tolerance in grapevines. J Exp Bot 2012; 63: 2127–2139.
Duan D, Halter D, Baltenweck R et al. Genetic diversity of stilbene metabolism in Vitis sylvestris. J Exp Bot 2015; 66: 3243–3257.
Vannozzi A, Dry IB, Fasoli M, Zenoni S . Genome-wide analysis of the grapevine stilbene synthase multigenic family: genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Biol 2012; 12: 1–22.
Höll J, Vannozzi A, Czemmel S, D'Onofrio C, Walker AR, Rausch T et al. The R2R3-MYB transcription factors MYB14 and MYB15 regulate stilbene biosynthesis in Vitis vinifera. Plant Cell 2013; 25: 4135.
Chang X, Nick P . Defence signalling triggered by Flg22 and harpin is integrated into a different stilbene output in vitis cells. PLoS ONE 2012; 7: e40446.
Jiao Y, Xu W, Dong D, Wang Y, Nick P . A stilbene synthase allele from a Chinese wild grapevine confers resistance to powdery mildew by recruiting salicylic acid signalling for efficient defence. J Exp Bot 2016; 67: 5841–5856.
Duan D, Fischer S, Merz P, Bogs J, Riemann M, Nick P . An ancestral allele of grapevine transcription factor MYB14 promotes plant defence. J Exp Bot 2016; 67: 1795–1804.
Xu W, Yu Y, Ding J, Hua Z, Wang Y . Characterization of a novel stilbene synthase promoter involved in pathogen- and stress-inducible expression from Chinese wild Vitis pseudoreticulata. Planta 2010; 231: 475–487.
Xu W, Yu Y, Zhou Q et al. Expression pattern, genomic structure, and promoter analysis of the gene encoding stilbene synthase from Chinese wild Vitis pseudoreticulata. J Exp Bot 2011; 62: 2745–2761.
Jaillon O, Aury JM, Noel B et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 2007; 449: 463–467.
Xu W, Jiao Y, Li R, Zhang N, Xiao D, Ding X et al. Chinese wild-growing Vitis amurensis ICE1 and ICE2 encode MYC-type bHLH transcription activators that regulate cold tolerance in Arabidopsis. PLoS ONE 2014; 9: e102303–e102303.
Wesley SV, Helliwell CA, Smith NA et al. Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J 2001; 27: 581–590.
Gleave AP . A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol 1992; 20: 1203–1207.
Nürnberger T, Brunner F, Kemmerling B, Piater L . Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev 2004; 198: 249–266.
Tena G, Asai T, Chiu WL, Sheen J . Plant mitogen-activated protein kinase signaling cascades. Curr Opin Plant Biol 2001; 4: 392–400.
Grant M, Brown I, Adams S, Knight M, Ainslie A, Mansfield J . The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. Plant J 2000; 23: 441–450.
Torres MA, Jones JDG, Dangl JL . Reactive oxygen species signaling in response to pathogens. Plant Physiol 2006; 141: 373–378.
Medina-Castellanos E, Esquivel-Naranjo EU, Heil M, Herrera-Estrella A . Extracellular ATP activates MAPK and ROS signaling during injury response in the fungus Trichoderma atroviride. Front Plant Sci 2014; 5: art. 659.
Pitzschke A, Hirt H . Mitogen-activated protein kinases and reactive oxygen species signaling in plants. Plant Physiol 2006; 141: 351–356.
Knight MR, Campbell AK, Smith SM, Trewavas AJ . Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 1991; 352: 524–526.
Miller EW, Chang CJ . Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc Natl Acad Sci USA 2010; 107: 15681.
Nürnberger T . Signal perception in plant pathogen defense. Cell Mol Life Sci 1999; 55: 167–182.
Buchanan BB, Gruissem W, Jones RL . Biochemistry & molecular biology of plants. American Society of Plant Physiologists 2000; 150: 171–172.
Qiao F, Chang XL, Nick P . The cytoskeleton enhances gene expression in the response to the Harpin elicitor in grapevine. J Exp Bot 2010; 61: 4021.
Chang X, Heene E, Qiao F, Nick P . The phytoalexin resveratrol regulates the initiation of hypersensitive cell death in Vitis cell. PLoS ONE 2011; 6: e26405.
Acknowledgements
This work was supported by the Key Laboratory of Horticultural Plant Biology and Germplasm Innovation in Northwest China. The research was funded by the National Science Foundation of China (Grant No. 31372039) and the National Science Foundation of China (Grant No. 31672129).
Author information
Authors and Affiliations
Contributions
YJW designed and initiated this research. YTJ, DW, LW and CYJ carried out the experiments and analyzed the results. YTJ wrote the manuscript, and YJW revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information for this article can be found on the Horticulture Research website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Jiao, Y., Wang, D., Wang, L. et al. VqMAPKKK38 is essential for stilbene accumulation in grapevine. Hortic Res 4, 17058 (2017). https://doi.org/10.1038/hortres.2017.58
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/hortres.2017.58
This article is cited by
-
Phytohormones mediated antifungal resistance against Fusarium oxysporum
Acta Physiologiae Plantarum (2024)
-
Genome-wide identification of MAPKKK genes and their responses to phytoplasma infection in Chinese jujube (Ziziphus jujuba Mill.)
BMC Genomics (2020)
-
Recent advances in biotechnological studies on wild grapevines as valuable resistance sources for smart viticulture
Molecular Biology Reports (2020)
-
The grapevine R2R3-type MYB transcription factor VdMYB1 positively regulates defense responses by activating the stilbene synthase gene 2 (VdSTS2)
BMC Plant Biology (2019)
-
Transcriptome signatures of tomato leaf induced by Phytophthora infestans and functional identification of transcription factor SpWRKY3
Theoretical and Applied Genetics (2018)









