Abstract
We report the case of a 22-year-old male with autosomal recessive Alport syndrome. Molecular analysis showed that this patient has a homozygous missense (NM_000091.4:c.3266G>A) Gly1089Asp mutation in the COL4A3 gene. The proband inherited the mutation from his heterozygous carrier mother, whereas the father carried only wild-type alleles. We performed comparative genome hybridization and single-nucleotide polymorphism microarray analyses and confirmed that there was partial maternal isodisomy.
Similar content being viewed by others
Alport syndrome (AS), the major cause of hereditary nephritis, is associated with sensorineural hearing loss, ocular abnormalities and ultrastructural abnormalities of the glomerular basement membrane, which causes persistent hematuria, proteinuria and end-stage renal failure. AS is due to defects in type IV collagen α3 (α3 [IV]), α4 (α4 [IV]) or α5 (α5 [IV]) chains, which are encoded by the COL4A3 (2q36-q37), COL4A4 (2q35-q37) and COL4A5 (Xq22) genes, respectively. The majority of AS (>80%) is inherited in an X-linked manner with mutations found in the COL4A5 gene,1 and autosomal recessive AS (ARAS) is caused by mutations in the COL4A3 or COL4A4 gene.2
Uniparental disomy (UPD) arises when a diploid individual carries either both homologs of a chromosomal pair from a single parent (uniparental heterodisomy) or two copies of a single parental chromosome (uniparental isodisomy).3 Isodisomy causes several autosomal recessive conditions, but there are few reports of isodisomy of the telomeric end of chromosome 2.4 Furthermore, there is no report of AS due to segmental UPD. This study sought to describe segmental maternal isodisomy of the telomeric end of chromosome 2, including the COL4A3 gene region, resulting in an ARAS phenotype in the patient.
The patient is a 22-year-old Japanese male who was born from nonconsanguineous parents. He presented with persistent proteinuria and microhematuria since the age of 2 years and a history of renal biopsy performed at age 7 years. Renal electron microscopic findings showed thinning of the glomerular basement membrane (Figure 1a). Immunohistochemical staining for type IV collagen α2 (α2 [IV]) expression was observed in the glomerular basement membrane, and staining for α5 [IV] was observed only in Bowman’s capsule (Figure 1b). These findings were compatible with typical ARAS findings. At the age of 17 years, he was diagnosed with progressive bilateral sensorineural hearing loss, and at 22 years, he underwent kidney transplantation. He has no other disorders, including physiological developmental delay. The patient’s mother had mild proteinuria but normal renal function. She had a normal audiogram and no ocular abnormalities. No other family member suffered from renal disease.
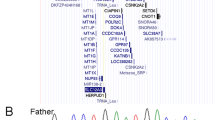
We performed genetic analysis of the patient to confirm the diagnosis. The sequences of the COL4A3, COL4A4 and COL4A5 genes were examined by direct DNA sequencing and showed a novel homozygous missense NM_000091.4:c.3266G>A, Gly1089Asp mutation in exon 39 of the COL4A3 gene. Genetic analysis of the mother showed that she is a heterozygous carrier of the mutation, whereas genetic analysis of the father showed only the wild-type sequence (Figure 2a). No mutations were found in the COL4A4 or COL4A5 genes. To rule out a hemizygotic mutation of this allele, we performed semiquantitative PCR analysis, which showed that the patient has two copies of the mutant COL4A3 gene. From these results, we considered the possibility of maternal isodisomy of chromosome 2, including the COL4A3 gene. To confirm the precise gene copy number and single-nucleotide polymorphism (SNP) haplotype of the region of the COL4A3 gene, microarray analysis using comparative genome hybridization (CGH) and SNP microarray (SurePrint G3 Human CGH+SNP Microarray 4×180 K, Agilent Technologies, Santa Clara, CA, USA) analysis were performed for the patient. SNP microarray data analysis for the patient revealed loss of heterozygosity (LOH) located in the chromosome region 2q33.3–2q37.2. The COL4A3 gene is located in this ~35 Mb LOH region (Figure 2b). The region of LOH was determined to be copy number neutral, with no gain or loss of genetic material. Comparative analysis of SNP genotyping data in the region of LOH confirmed the occurrence of maternal isodisomy. This finding strongly suggests a segmental uniparental isodisomy of maternal origin in this region that includes the COL4A3 gene. To confirm UPD, we tested polymorphisms using 16 single-nucleotide microsatellite markers spanning the entire length of chromosome 2. Nine markers were uninformative because they could have been inherited from either parent. Four other markers showed a heterozygous pattern compatible with a biparental mode of inheritance. Three markers showed a homozygous maternal inheritance, suggesting that reduction to homoallelism for the mutant COL4A3 gene allele was due to segmental maternal isodisomy of the telomeric end of chromosome 2. These results confirmed that the patient has ARAS due to maternal isodisomy. Finally, we determined that a homozygous mutation in the COL4A3 gene was caused by nonMendelian inheritance with segmental maternal isodisomy of the telomeric end of chromosome 2.
The family pedigree and sequencing of the COL4A3 gene show a homozygous missense Gly1089Asp mutation in the patient. His mother is a carrier, whereas his father does not carry the mutation (a). Agilent CytoGenomics Analysis Software shows that there is no 2q deletion, and LOH is detected in the telomeric end of chromosome 2 (chr2: 207,541,513–243,014,630; b). CGH, comparative genome hybridization; SNP, single-nucleotide polymorphism; WT, wild type.
The present case is the first reported case of AS due to partial segmental UPD and is the second reported case of segmental maternal isodisomy of the telomeric end of chromosome 2q. The first report described reduction to homoallelism for a primary hyperoxaluria type 1 mutation in the case of a patient with complete liver alanine:glyoxylate aminotransferase deficiency with no symptoms, but liver and kidney dysfunction.5 Although many cases of UPD—such as Beckwith–Wiedemann syndrome (UPD11) or Prader–Willi syndrome (UPD15)—are related to genomic imprinting and show various phenotypes, including intellectual disability, the present case shows typical ARAS. This outcome may stem from the fact that there are no imprinting regions in chromosome 2.6 Because the recurrence risk after the birth of a child with segmental UPD seems to be negligible,7 our result is useful for genetic counseling of the family.
We confirmed that there is a homozygous missense Gly1089Asp mutation of the COL4A3 gene in the patient. Although this mutation has not been reported in ARAS patients, the PolyPhen-2 score (http://genetics.bwh.harvard.edu/pph2/) is 1.000, which indicates ‘probably damaging,’ and glycine substitutions in the collagenous domain of the COL4A3 gene lead to the crucial constitutional changes resulting in the development of renal abnormalities. Although various genetic abnormalities of AS, including missense mutation, nonsense mutation, splicing error, nucleotide deletion and/or insertion, have been reported,8 to the best of our knowledge, the present case is the first case of a patient with AS due to UPD. We have also reported the cases of 30 Japanese ARAS patients,9 and among this group, there were no UPD patients other than the present patient (who is Patient number 114 in the literature).
The mechanisms of monosomy or trisomy rescue result in complete UPD, whereas segmental isodisomy with a normal status on the rest of chromosome as observed in this study indicates a fusion of maternal and paternal chromosomes, and was therefore most likely due to a postzygotic event.3 Somatic recombination may be the possible mechanism for the segmental isodisomy. In this patient, homologous recombination between paternal and maternal chromatids may have occurred in the very early postzygotic period, as the vast majority of the patient’s cells were found to carry the homozygous mutation based on the sequence data. Homologous recombination is one of the mechanisms for the repair of double-strand breaks. In this patient, the region of segmental isodisomy extends to the end of the long arm of chromosome 2, suggesting that break-induced replication, one of the double-strand break repair pathways similar to homologous recombination, was likely to lead to the generation of the large segmental isodisomy.10
In conclusion, this is the first reported case of a patient with AS due to UPD. Our observations may lead to an improved understanding of the genetic polymorphism of AS.
References
References
Martin P, Heiskari N, Zhou J, Leinonen A, Tumelius T, Hertz JM et al. High mutation detection rate in the COL4A5 collagen gene in suspected Alport syndrome using PCR and direct DNA sequencing. J Am Soc Nephrol 1998; 9: 2291–2301.
Mochizuki T, Lemmink HH, Mariyama M, Antignac C, Gubler MC, Pirson Y et al. Identification of mutations in the alpha 3(IV) and alpha 4(IV) collagen genes in autosomal recessive Alport syndrome. Nat Genet 1994; 8: 77–81.
Engel E . A new genetic concept: uniparental disomy and its potential effect, isodisomy. Am J Med Genet 1980; 6: 137–143.
Herzfeld T, Wolf N, Winter P, Hackstein H, Vater D, Müller U . Maternal uniparental heterodisomy with partial isodisomy of a chromosome 2 carrying a splice acceptor site mutation (IVS9-2A>T) in ALS2 causes infantile-onset ascending spastic paralysis (IAHSP). Neurogenetics 2009; 10: 59–64.
Chevalier-Porst F, Rolland MO, Conchat P, Bozon D . Maternal isodisomy of the telomeric end of chromosome 2 is responsible for a case of primary hyperoxaluria type 1. Am J Med Genet A 2005; 132A: 80–83.
Baskin B, Geraghty M, Ray PN . Paternal isodisomy of chromosome 2 as a cause of long chain 3-hydroxyacyl-CoA dehydrogenase [LCHAD] deficiency. Am J Med Genet A 2010; 152A: 1808–1811.
Kotzot D . Complex and segmental uniparental disomy updated. J Med Genet 2008; 45: 545–556.
Storey H, Savige J, Sivakumar V, Abbs S, Flinter FA . COL4A3/COL4A4 mutations and features in individuals with autosomal recessive Alport syndrome. J Am Soc Nephrol 2013; 24: 1945–1954.
Oka M, Nozu K, Kaito H, Fu XJ, Nakanishi K, Hashimura Y et al. Natural history of genetically proven autosomal recessive Alport syndrome. Pediatr Nephrol (e-pub ahead of print 15 March 2014; 10.1007/s00467-014-2797-4).
Chen JM, Cooper DN, Férec C, Kehrer-Sawatzki H, Patrinos GP . Genomic rearrangement in inherited disease and cancer. Semin Cancer Biol 2010; 20: 222–233.
Data Citations
Morisada, Naoya HGV Database http://hgv.figshare.com/genome_variation/13 (2014)
Acknowledgements
All phases of this study were supported by a grant from the Ministry of Health, Labour and Welfare (Japan) for Research on Rare Intractable Diseases in Kidney and Urinary Tract (H24-nanchi-ippan-041 to KI) in the ‘Research on Measures for Intractable Diseases’ Project, and a Grant-in-Aid for Scientific Research (KAKENHI) from the Ministry of Education, Culture, Sports, Science and Technology (Subject ID: 25893131 to KN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Fu, X., Morisada, N., Hashimoto, F. et al. A patient with autosomal recessive Alport syndrome due to segmental maternal isodisomy. Hum Genome Var 1, 14006 (2014). https://doi.org/10.1038/hgv.2014.6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/hgv.2014.6
This article is cited by
-
Alport syndrome: deducing the mode of inheritance from the presence of haematuria in family members
Pediatric Nephrology (2020)