Abstract
The main objectives of this study were to test: (1) whether the W-chromosome differentiation matches to species’ evolutionary divergence (phylogenetic concordance) and (2) whether sex chromosomes share a common ancestor within a congeneric group. The monophyletic genus Triportheus (Characiformes, Triportheidae) was the model group for this study. All species in this genus so far analyzed have ZW sex chromosome system, where the Z is always the largest chromosome of the karyotype, whereas the W chromosome is highly variable ranging from almost homomorphic to highly heteromorphic. We applied conventional and molecular cytogenetic approaches including C-banding, ribosomal DNA mapping, comparative genomic hybridization (CGH) and cross-species whole chromosome painting (WCP) to test our questions. We developed Z- and W-chromosome paints from T. auritus for cross-species WCP and performed CGH in a representative species (T. signatus) to decipher level of homologies and rates of differentiation of W chromosomes. Our study revealed that the ZW sex chromosome system had a common origin, showing highly conserved Z chromosomes and remarkably divergent W chromosomes. Notably, the W chromosomes have evolved to different shapes and sequence contents within ~15–25 Myr of divergence time. Such differentiation highlights a dynamic process of W-chromosome evolution within congeneric species of Triportheus.
Similar content being viewed by others
Introduction
Sex chromosomes are thought to have evolved from an autosomal pair when a sex-determining region or locus evolves on one of the homologs (Bull, 1983; Charlesworth, 1991). Interaction of sex-determining region or locus with sexually antagonistic polymorphisms maintained in linked genes is thought to have favored recombination suppression between the nascent sex chromosomes (Bachtrog, 2006) and the subsequent acquisition of neutral and deleterious mutations (genetic degeneration), amplification of repetitive DNA sequences and heterochromatinization of the sex-specific chromosome (Charlesworth et al., 2005; Bachtrog, 2006). Therefore, differences in size and gene content can be found among the sex chromosomes, in which the Y (or W) may undergo variable degrees of degeneration (Graves, 2006).
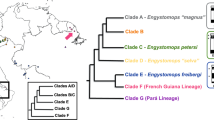
Here we study sex chromosome evolution in a fish genus. In teleost fishes, although most species lack heteromorphic sex chromosomes, a variety of sex chromosome systems including simple and multiple ones can be found in some species (Devlin and Nagahama, 2002; Cioffi et al., 2011). Among the ∼10% of teleost fish species studied that have detectably heteromorphic sex chromosomes, most have female heterogamety (Devlin and Nagahama, 2002). We studied one such group, the genus Triportheus (Characiformes, Triportheidae) with ZW sex chromosomes in 12 species so far analyzed. The Z is a metacentric chromosome, the largest one in the karyotype, whereas the W is always smaller than the Z, and varies in size and morphology among species (Bertollo and Cavallaro, 1992; Sánchez and Jorge, 1999; Artoni et al., 2001; Artoni and Bertollo, 2002; Nirchio et al., 2007; Diniz et al., 2008; Yano et al., 2014, 2016). Besides, the W chromosome is rich in heterochromatin and carries an 18S ribosomal DNA (rDNA) site on its long arms (Artoni and Bertollo, 2002; Nirchio et al., 2007; Diniz et al., 2009; Marquioni et al., 2013; Yano et al., 2014; Schmid et al., 2016). Recently, a molecular phylogeny of the Triportheidae family was introduced based on the 16S rRNA and cytochrome b (CytB) mitochondrial genes, and on the recombination activating gene 1 (Rag1), recombination activating gene 2 (Rag2) and myosin heavy chain 6 cardiac muscle-α (Myh6) nuclear genes (Mariguela et al., 2016). According to this study, Triportheus represent a monophyletic group originated at 26.2±6.5 Myr, in which T. auritus is a direct representative of the first lineage that differentiated in the genus at 20.7±6.5 Myr, and correspond to a sister group to all Triportheus species (Mariguela et al., 2016), as demonstrated in the phylogenetic tree (Figure 1).
Adapted phylogenetic tree for the Triportheus genus, based on the phylogenetic data generated by Mariguela et al. (2016), with the respective C-banded Z and W chromosomes evidencing the divergence in the size of the latter. Besides the species analyzed in this study, T. venezuelensis and T. angulatus (indicated in asterisks) were also included. Note that T. auritus, which corresponds to a sister group to all Triportheus species, carries the larger W chromosome, whereas T. albus has the smaller one compared with its congeneric species.
Molecular cytogenetics provides valuable tools and insights for comparative genomics research and has emerged as promising for understanding genome evolution and organization. In particular, whole chromosome painting (WCP) and comparative genomic hybridization (CGH) have been effective methods for the identification and characterization of sex chromosomes, tracking their origin and evolution among various taxa (Traut et al., 1999; Phillips et al., 2001; Ezaz et al., 2005; Henning et al., 2008; Ráb et al., 2008; Cioffi et al., 2013; Pazian et al., 2013; Symonová et al., 2015). However, the effectiveness of CGH technique can be limited to identify nascent sex chromosomes with very small sex-specific sequences, as demonstrated in the iguana Oplurus fierinensis (Altmanová et al., 2016).
In a diversity of organisms, heterochromatinization, accompanied by amplification of tandem repeats, represents an important step in the morphological differentiation of simple sex chromosome systems, especially in the ZW ones (Nanda et al., 2000; Kondo et al., 2004; Marchal et al., 2004; Peichel et al., 2004; Charlesworth et al., 2005; Ezaz et al., 2009; Kejnovský et al., 2009). Same type of studies provided evidence that rDNA cistrons can also occur on the sex chromosomes of distinct organisms (see, for example, Goodpasture and Bloom, 1975; Schmid et al., 1983; Yonenaga-Yassuda et al., 1983; Morielle and Varella-Garcia, 1988; Cioffi et al., 2010). In this sense, the detection of the heterochromatin by C-banding procedures, as well as the mapping of rDNA repeats, represent helpful approaches for sex chromosome identification and characterization.
Here we compared sex chromosomes of eight species from Triportheus genus using multiple molecular and conventional cytogenetic tools, such as C-banding to detect heterochromatin, rDNA mapping, CGH and WCP to characterize regions of homology between the Z and W chromosomes, which we assume represent the ancestral state, as well as the size of the W-specific region. Our study confirmed that the Z chromosomes are highly conserved and revealed remarkably divergent W-chromosome shapes and sequence content.
Materials and methods
Fish species and sample collection
Table 1 lists the individuals investigated, the collection locations, sexes and the numbers of cells analyzed in the cytogenetic experiments. Collections had the authorization of the Brazilian environmental agency ICMBIO/SISBIO (License number 48628-2). All species were identified and deposited in the fish museum of the Laboratory of Biology and Genetic of Fishes of the Universidade Estadual Paulista (UNESP; Botucatu, Brazil) (Table 1). The experiments followed ethical and anesthesia rules in accordance with the Ethics Committee on Animal Experimentation of the Universidade Federal de São Carlos (Process number CEUA 1853260315).
Chromosome preparations and C-banding
Mitotic chromosomes were obtained as described in Bertollo et al. (2015). Briefly, the animals were treated with an aqueous solution of colchicine for 50–60 min, anesthetized and killed, and the chromosomal preparations were obtained from cells of the anterior kidney. The C-positive heterochromatin was detected using barium hydroxide according to Sumner (1972).
Chromosome microdissection, probe preparation and fluorescence in situ hybridization (FISH)
Fifteen copies of the Z and 20 copies of the W chromosomes from T. auritus were microdissected, as it corresponds to a sister group to all Triportheus species (Mariguela et al., 2016) and harbors the largest W chromosome. The chromosomes were manually microdissected and pooled before amplifying by degenerate oligonucleotide primed-PCR, following the protocol described in Telenius et al. (1992). Chromosome paints were prepared following Yang et al. (2009). The Z probes were labeled via PCR with SpectrumOrange-dUTP (Vysis, Downers Grove, IL, USA) and the W probes with SpectrumGreen-dUTP (Vysis) in a 30-cycle label PCR with degenerate oligonucleotide primer using 1 μl of the primary degenerate oligonucleotide primed-PCR products as template DNA (Yang et al., 2009). 18S and 5S rDNA probes were obtained according to Cioffi et al. (2009) and Martins et al. (2006), respectively. The 18S rDNA probe was labeled with Cyanine 5-dUTP, whereas 5S rDNA was labeled with Spectrum Green-dUTP using nick-translation method (Roche, Mannheim, Germany).
Three-color FISH for WCP
Cytogenetic preparations of males and females of the eight mentioned Triportheus species were used for a three-color FISH experiment, combining microdissected Z and W chromosomes, together with 18S rDNA probe, according to Yang et al. (2009). As commercial salmon sperm blocking DNA (Sigma-Aldrich, St Louis, MO, USA) was not sufficient to block the hybridization of high-copy repeat sequences, therefore, Cot1-DNA directly isolated from T. auritus female genome (prepared according to Zwick et al., 1997) was used instead. Hybridization was performed for 16–18 h at 37 °C in a moist chamber. After hybridization, the slides were washed for 5 min with 1 × SSC at 65 °C, and in 4 × SSC/Tween using a shaker at room temperature and then rinsed quickly in 1 × phosphate-buffered saline. Subsequently, the slides were dehydrated again in an ethanol series (70, 85 and 100%) for 2 min each. After complete drying of the slides, the chromosomes were counterstained with DAPI/Antifading (1.2 mg ml−1, Vector Laboratories, Burlingame, CA, USA).
Preparation of probes for CGH
The CGH experiments were performed in T. signatus, representing a species with one of the smallest W chromosomes found among Triportheus species. The female genomic DNA (gDNA) was labeled with digoxigenin-11-dUTP using DIG-nick-translation Mix (Roche), and the male gDNA was labeled with biotin-16-dUTP using BIO-nick-translation Mix (Roche), in which 1 μg of gDNA was used, each. Hybridization mixture for one slide (25–30 μl) was composed of 1 μg of labeled male gDNA, 1 μg of labeled female gDNA and 50 μg of sonicated salmon sperm blocking DNA (Sigma-Aldrich).
FISH for CGH
The CGH experiments followed the methodology described by Symonová et al. (2015). The hybridization signal was detected using a solution composed of anti-digoxigenin-fluorescein isothiocyanate (Roche) diluted in 0.5% bovine serum albumin in phosphate-buffered saline, and streptavidin-CY3 (Invitrogen Life Technologies, San Diego, CA, USA) diluted in phosphate-buffered saline containing 10% normal goat serum. The slides were then washed 4 times in 4 × SSC and 0.01% Tween, 7 min each at 42 °C. After complete drying, the chromosomes were counterstained and mounted in antifade containing 1.5 μg ml−1 DAPI (Cambio, Cambridge, UK).
Two-color FISH with 18S and 5S rDNA probes
18S and 5S rDNA sequences were mapped on female chromosome preparations of T. signatus species, following the protocol described in Marquioni et al. (2013). For this experiment, the 18S and 5S rDNA probes were labeled with SpectrumOrange-dUTP and SpectrumGreen-dUTP, respectively, using nick-translation method (Roche).
Microscopic analyses
At least 20 metaphase spreads were analyzed per individual to confirm the diploid chromosome numbers, karyotype structure and FISH results. Images were captured by the CoolSNAP system software, Image Pro Plus, 4.1 (Media Cybernetics, Silver Spring, MD, USA), coupled to an Olympus BX50 microscope (Olympus Corporation, Ishikawa, Japan).
Results
C-banding and rDNA mapping
C-positive heterochromatin was consistently localized in the centromeric regions of autosome pairs (data not shown). The Z chromosomes have additional heterochromatin in one or both telomeric regions, depending on the species, whereas the W chromosomes were almost entirely heterochromatic, except for the p arms in all species (Figure 1), in agreement with previous studies (Artoni and Bertollo, 2002; Diniz et al., 2009; Yano et al., 2014).
The two-color FISH with 18S and 5S rDNA showed that both sites are colocalized on the p arms of the chromosome pair 3 in T. signatus, with an 18S rDNA additional site adjacent to the Wq telomere (Figure 2).
CGH on female metaphase of T. signatus, with emphasis on the Z and W chromosomes. The superposition of male and female gDNA probes highlights chromosomal regions sharing male and female sequences and the prevalence of female- or male-specific sequences. (a) DAPI staining. (b) Hybridization with gDNA female probe. (c) Hybridization with gDNA male probe. (d) Superposition of female and male gDNA probes. In (e), the sex chromosomes after the superposition of female and male gDNA probes are highlighted in enlarged forms, together with schematic diagrams summarizing the results, evidencing the accumulation of female-specific sequences in the terminal region of the Wq. The chromosome pair 3 and the W chromosome harboring 18S and 5S rDNA are boxed. Bar, 5 μm.
Comparative genomic hybridization
CGH using male and female gDNA probes developed from T. signatus identified Z- and W-specific sequences. In females, all chromosomes except the W, stained equally with these probes. The female gDNA probe painted the whole W chromosome, with a very bright signal on most of the Wq, as well as the telomeric region of both Z chromosome arms. In addition, the p arms of pair 3 also showed an extensive homology with the Wq region. With the male gDNA probe, the W chromosome showed signals on Wp and the proximal region of Wq. The merged images revealed that sequences from both sexes are shared on Wp and the proximal region of the Wq arms, whereas female-specific sequences are concentrated in the terminal region of the Wq (Figure 2).
Sex chromosome paint preparation and cross-species sex chromosome painting
The quality of both Z- and W-chromosome paints was validated by mapping them back onto T. auritus metaphase spreads with salmon sperm DNA and T. auritus female-specific Cot1-DNA as suppressor. The probe mixed with salmon sperm DNA produced nonspecific signals (data not shown), whereas 5 μg μl−1 of T. auritus female-specific Cot1-DNA was sufficient to block nonspecific signals, giving clear hybridization signals on Z and W chromosomes, and identifying probes that are largely or completely Z or W specific (Figures 3 and 4).
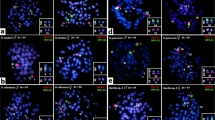
Cross-species chromosome painting using W-chromosome (green) and Z-chromosome (red) probes, both obtained from T. auritus, together with an 18S rDNA probe (yellow) in a three-color FISH experiment. The sex chromosomes are highlighted in boxes. Note the location of 18S rDNA sequences on the telomeric Wq. The additional 18S rDNA sites located on autosomes are not shown. Bar, 5 μm. A full color version of this figure is available at the Heredity journal online.
Hybridization patterns on the sex chromosomes of Triportheus species using Z- and W-chromosome probes. (a) Note that the sex chromosomes showed evident FISH signals with both Z and W probes, although with a variable pattern among species. In (b), the diagrams explaining the observed hybridization patterns, taking account of the use of Cot11-DNA from a female of T. auritus as a competitor. The W-specific regions (terminal part of the q arms) were thus blocked and did not hybridize. Accordingly, on the euchromatic Z chromosome, only the centromeric highly repetitive region and the ancestral homologous region (terminal part of the p arms) were painted with the W probe. In (c), ZW chromosomes after CGH experiments performed in T. signatus are highlighted.
Together with the Z and W probes, an additional 18S rDNA probe was used in a three-color FISH experiment to clearly identify the W chromosome in the metaphase plates, as the W chromosome carries a huge 18S rDNA cistron in the Wq arms of all Triportheus species. The Z probe from T. auritus completely painted the Z chromosome of all other species. The W chromosome of this species also displayed fluorescence signals in the p arms, but there were remarkable differences among species, with only T. guenteri, T. nematurus and T. albus showing strong hybridization signals across the whole Wp. A major part of the Wq arms was also painted with the Z probe in all species, with the exception of T. aff. rotundatus and pantanensis (Figures 3 and 4).
A high level of homology was found for the W chromosome among species, but with different hybridization patterns (Figures 3 and 4). Using the W probes, the W chromosome was homogeneously painted along its entire length in all species, except for the Wq telomeric region and the centromeric region of T. aff. rotundatus and T. pantanensis (Figures 3 and 4). In all species, the W probe also painted the Z chromosome in the centromeric and telomeric regions of the p arms, with the exception of T. auritus, in which only the telomeric region was painted (Figures 3 and 4). As the whole chromosome painting experiments were performed using a T. auritus female Cot1-DNA as a competitor, the W-specific region (terminal part of the q arms) was thus blocked and did not hybridize. Accordingly, on the euchromatic Z chromosome, only the centromere (a highly repetitive region) and the ancestral homologous region (terminal part of the p arms) were painted with the W probe, with the exception of the centromeric region of the Z chromosome of T. auritus that was also blocked or suppressed (Figure 4).
Discussion
Our study demonstrated that the ZW sex chromosome system in Triportheus had a common origin, considering the homology found in all species using the Z- and W-chromosome probes from T. auritus; however, the W chromosomes have undergone rapid discordant differentiation, as differential hybridization patterns were verified for these chromosomes in WCP experiments. In fact, the W chromosomes display a differential molecular composition, size and morphology among species despite their evolutionary relationships, highlighting the dynamic process that shapes the differentiation of the sex chromosomes. In addition, our study provided molecular cytogenetic evidence of the chromosomal rearrangement involving rDNA locus, highlighting its probable role in the evolution of the sex chromosomes by facilitating the reduced recombination and the subsequent accumulation of repetitive sequences on the W chromosome that ultimately led the evolution of highly differentiated sex chromosomes within this congeneric group.
Chromosomal rearrangements and sex chromosome differentiation
It is clear that size reduction and accumulation of heterochromatin are events that are associated with the differentiation of the W chromosome within Triportheus species (Figure 1). However, CGH and WCP experiments were able to clarify additional details on this evolutionary pathway. In fact, both techniques were critical to demonstrate the sequences that are still shared by both sex chromosomes or, otherwise, that those are more exclusive to one of them. Noteworthy, it was evidenced that the end of the Wq has a high concentration of female-specific sequences (Figures 2–4), where an 18S rDNA cluster is also located in all Triportheus species so far analyzed (Artoni and Bertollo, 2002; Nirchio et al., 2007; Diniz et al., 2009; Marquioni et al., 2013; Yano et al., 2014). Sex chromosomes carrying 18S rDNA sequences have already been reported in several other vertebrates, such as Characidium fishes (Scacchetti et al., 2015), cane toad Bufo marinus (Abramyan et al., 2009), Chinese softshell turtle Pelodiscus sinensis (Kawai et al., 2007) and tiger snake Notechis scutatus (O’Meally et al., 2010). However, the Triportheus case deserves further considerations because of the unusual and particular location of these sequences that do not occur in both homologs of the sex pair (only on the W chromosome) and in some autosomes.
Unusually, the Triportheus sex chromosomes do not have 18S rDNA sequences on both homologs, but they are present only on the W chromosome. A huge 18S rDNA block is also located on the p arms of the third chromosome pair in all Triportheus species. Besides, in some species additional 18S rDNA sites are verified in other autosomes (Yano et al., submitted). The 18S rDNA region of the third chromosome also showed high homology with the Wq, in our CGH experiments, suggesting that either part of the 18S rDNA block was transposed from the W chromosome to the 3p arms or vice versa. Evidence for an 18S rDNA cluster in the telomeric region of the Z chromosome was reported in T. venezuelensis and T. angulatus (Nirchio et al., 2007; Marquioni et al., 2013) that represent two of the most recently derived species, as their lineage originated at 5.2±2.3 and 2.6±1.4 Myr, respectively (Mariguela et al., 2016). Considering the fact that most species, including those originated from older Triportheus lineages, do not show an 18S rDNA cluster on the Z chromosome, it is more plausible to assume that these sequences were firstly translocated onto W and latter transposed from the W to the Z chromosome in T. venezuelensis and T. angulatus in independent events. However, an alternative scenario in which these sequences were originally carried on both sex chromosomes, and that their present distribution reflects subsequent loss from the Z in some species, cannot be fully excluded.
It is tempting to speculate that maintenance and amplification of the rDNA sites on the W chromosome might have promoted reduced recombination between the ZW pair (see Figure 5). It has been proposed that polymorphisms in the rDNA locus in Salvelinus species may have acted in a similar way to limit crossing over near the sex locus (Reed and Phillips, 1997). Besides 18S rDNA, the W chromosome of Triportheus is rich in other repetitive DNA classes, as evidenced by the variable accumulation of microsatellites and U2 snDNA (Yano et al., 2016; Yano et al., submitted). Therefore, we cannot rule out the possible involvement of distinct classes of repetitive DNA sequences in the differentiation process of W chromosome. Our WCP and CGH experiments clearly demonstrate that much of the Wq is a W-specific region (Figures 2–4), but that differentiation of the W chromosome morphology, associated with heterochromatinization, and probably with degeneration, evolved independently in different Triportheus lineages (Figure 5). These changes are specific to the W chromosome, whereas other chromosomes, including Z, remain similar in the Triportheus species so far analyzed, supporting the monophyletic status of this genus, but indicating that the female-specific sex chromosome is subject to particular evolutionary forces not shared by other chromosomes. Moreover, as the sex-specific chromosome can experience different evolutionary forces, parameters like effective population size and sexual selection may also affect how the recombination suppression evolves (Graves, 2006; Bachtrog et al., 2011), even among closely related species as in the Triportheus case.
Schematic diagram summarizing the proposed chromosome rearrangements leading to the evolution of sex chromosomes in Triportheus species. (a) Fission and translocation event involving the p arms of an ancestral chromosome 3 occurred within an 18S rDNA cluster onto the W chromosome, or vice versa, in a common ancestor. (b) Subsequently, such 18S rDNA cistron was amplified in W chromosome. (c) Finally, whereas the Z chromosome was morphologically conserved, the W chromosome had extensive morphological and size variations because of rDNA expansion, heterochromatinization and repetitive DNA accumulation, in which the degree of degeneration and the differentiation later evolved independently in each Triportheus species. W1, T. auritus; W2, T. guentheri; W3, T. signatus; W4, T. rotundatus; W5, T. nematurus; W6, T. pantanensis; W7, T. trifurcatus; W8, T. venezuelensis (data from Nirchio et al., 2007); W9, T. angulatus (data from Diniz et al., 2009); W10, T. albus.
Common origin of the ZW sex system in Triportheus and differentiation of the W chromosome
The Triportheidae has four other genera, including Lignobrycon, in which L. myersi represents the only species currently described and corresponds to the sister group of all other Triportheidae species (Mariguela et al., 2016). Recent analyses indicate a similar ZW sex chromosome system in L. myersi (Rodrigues et al., 2016), suggesting an early origin of the ZW system in the family, an unusual situation among fishes, where sex chromosomes have evolved independently among congeneric species, or even within the same species (Cioffi et al., 2013).
The molecular divergence, demonstrated by cross-species WCP, and the variable morphological forms, underlines the process shaping the evolution of the sex-specific chromosome in Triportheus. Rapid degeneration of recently formed Y chromosomes has been demonstrated in Drosophila (Bachtrog et al., 2008) and sticklebacks (Peichel et al., 2001). However, there are few empirical data for W chromosomes. The data presented here suggest that the W chromosomes of Triportheus have evolved during a relative short (~15–25 Myr) divergence time (phylogenetic data from Mariguela et al., 2016), displaying different genomic composition in terms of repetitive DNA sequences, size, and morphology among species, including accumulation of microsatellites, transposable elements and rDNAs on the W chromosomes (Yano et al., 2014), like the situation in the W chromosomes of pyralid moths (Vítková et al., 2007).
The current situation in Triportheus resembles the sex chromosome evolution in other taxa, such as birds and snakes. In primitive ratite birds, the W chromosome is almost the same size as the Z (Shetty et al., 1999), and this may represent the ancestral condition from which other bird W chromosomes have evolved (Ferguson-Smith, 2007). Similarly, T. auritus, the earliest branching extant Triportheus species (Mariguela et al., 2016), has the largest W chromosome, with a size comparable to the Z (Figure 1). However, we detect no correlation between the size reduction of the W chromosome and the divergence time between Triportheus species (Figure 1). Similarly, various bird species also show variations in the morphology of the W chromosome, but no clear pattern of gradual reduction in size over time (Rutkowska et al., 2012).
Conclusions
Overall, our study provides evidence for the common origin of a sex chromosome system within a congeneric group that corresponds to an uncommon event among fish species, even among closely related ones, where independent evolution is more common. It is also remarkable that rapid differentiation of the sex-specific chromosome occurred in size, shape and sequence content, likely favored by recombination reduction between the sex pair in view of the maintenance and amplification of specific genome sequences in the W chromosome. Our study provides a unique opportunity for fine-scale analysis of sex chromosome sequences in this group through sequencing and sequence analysis of microdissected sex chromosomes that will assist in discovering novel sex-determining genes and mechanisms of sex chromosome evolution in vertebrates in general.
Data archiving
Description of the sampling sites and their GPS coordinates can be found at the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.8m201.
References
Abramyan J, Ezaz T, Graves JAM, Koopman P . (2009). Z and W sex chromosomes in the cane toad (Bufo marinus). Chromosome Res 17: 1015–1024.
Altmanová M, Rovatsos M, Kratochvíl L, Pokorná MJ . (2016). Minute Y chromosomes and karyotype evolution in Madagascan iguanas (Squamata: Iguania: Opluridae). Biol J Linn Soc 118: 618–633.
Artoni RF, Bertollo LAC . (2002). Evolutionary aspects of the ZZ/ZW sex chromosome system in the Characidae fish, genus Triportheus. A monophyletic state and NOR location on the W chromosome. Heredity 89: 15–19.
Artoni RF, Falcão JN, Moreira-Filho O, Bertollo LAC . (2001). An uncommon condition for a sex chromosome system in Characidae fish. Distribution and differentiation of the ZZ/ZW system in Triportheus. Chromosome Res 9: 449–456.
Bachtrog D . (2006). A dynamic view of sex chromosome evolution. Curr Opin Genet Dev 16: 578–585.
Bachtrog D, Hom E, Wong K, Maside X, de Jong P . (2008). Genomic degradation of a young Y chromosome in Drosophila miranda. Genome Biol 9: R30.
Bachtrog D, Mank JE, McDaniel SF, Pires JC, Rice W, Valenzuela N . (2011). Are all sex chromosomes created equal? Trends Genet 27: 350–357.
Bertollo LAC, Cavallaro ZI . (1992). A highly differentiated ZZ/ZW sex chromosome system in a Characidae fish, Triportheus guentheri. Cytogenet Cell Genet 60: 60–63.
Bertollo LAC, Cioffi MB, Moreira-Filho O . (2015) Direct chromosome preparation from Freshwater Teleost Fishes. In: Ozouf-Costaz C, Pisano E, Foresti F, Almeida Toledo LF (eds), Fish Cytogenetic Techniques (Chondrichthyans and Teleosts). CRC Press: Enfield. pp 21–26.
Bull JJ . (1983) Evolution of Sex Determining Mechanisms. Benjamin/Cummings Publishing Company: Menlo Park.
Charlesworth B . (1991). The evolution of sex chromosomes. Science 251: 1030–1033.
Charlesworth D, Charlesworth B, Marais G . (2005). Steps in the evolution of heteromorphic sex chromosomes. Heredity 95: 118–128.
Cioffi MB, Camacho JPM, Bertollo LAC . (2011). Repetitive DNAs and differentiation of sex chromosomes in neotropical fishes. Cytogenet Genome Res 132: 188–194.
Cioffi MB, Liehr T, Trifonov V, Molina WF, Bertollo LAC . (2013). Independent sex chromosome evolution in lower vertebrates: a molecular cytogenetic overview in the Erythrinidae fish family. Cytogenet Genome Res 141: 186–194.
Cioffi MB, Martins C, Vicari MR, Rebordinos L, Bertollo LAC . (2010). Differentiation of the XY sex chromosomes in the fish Hoplias malabaricus (Characiformes, Erythrinidae): unusual accumulation of repetitive sequences on the X chromosome. Sex Dev 4: 176–185.
Cioffi MB, Martins C, Centofante L, Jacobina U, Bertollo LAC . (2009). Chromosomal variability among allopatric populations of Erythrinidae fish Hoplias malabaricus: mapping of three classes of repetitive DNAs. Cytogenet Genome Res 125: 132–141.
Devlin RH, Nagahama Y . (2002). Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208: 191–364.
Diniz D, Laudicina A, Bertollo LAC . (2009). Chromosomal location of 18S and 5S rDNA sites in Triportheus fish species (Characiformes, Characidae). Genetic Mol Biol 32: 37–41.
Diniz D, Moreira-Filho O, Bertollo LAC . (2008). Molecular cytogenetics and characterization of a ZZ/ZW sex chromosome system in Triportheus nematurus (Characiformes, Characidae). Genetica 133: 85–91.
Ezaz T, Moritz B, Waters PD, Graves JAM, Georges A, Sarre SD . (2009). The ZW sex microchromosomes of an Australian dragon lizard share no homology with those of other reptiles or birds. Chromosome Res 17: 965–973.
Ezaz T, Quinn AE, Miura I, Sarre SD, Georges A, Graves JAM . (2005). The dragon lizard Pogona vitticeps has ZZ/ZW micro-sex chromosomes. Chromosome Res 13: 763–776.
Ferguson-Smith M . (2007). The evolution of sex chromosomes and sex determination in vertebrates and the key role of DMRT1. Sex Dev 1: 2–11.
Goodpasture C, Bloom SE . (1975). Visualization of nucleolar organizer regions in mammalian chromosomes using silver staining. Chromosoma 53: 3740.
Graves JAM . (2006). Sex chromosome specialization and degeneration in mammals. Cell 124: 901–914.
Henning F, Trifonov V, Ferguson-Smith MA, Almeida-Toledo LF . (2008). Non-homologous sex chromosomes in two species of the genus Eigenmannia (Teleostei: Gymnotiformes). Cytogenet Genome Res 121: 55–58.
Kawai A, Nishida-Umehara C, Ishijima J, Tsuda Y, Ota H, Matsuda Y . (2007). Different origins of bird and reptile sex chromosomes inferred from comparative mapping of chicken Z-linked genes. Cytogenet Genome Res 117: 92–102.
Kejnovský E, Hobza R, Čermak T, Kubat Z, Vyskot B . (2009). The role of repetitive DNA in structure and evolution of sex chromosomes in plants. Heredity 102: 533–541.
Kondo M, Nanda I, Hornung U, Schmid M, Schartl M . (2004). Evolutionary origin of the medaka Y chromosome. Curr Biol 14: 1664–1669.
Marchal JA, Acosta MJ, Nietzel H, Sperling K, Bullejos M, Díaz de la Guardia R et al. (2004). X chromosome painting in Microtus: origin and evolution of the giant sex chromosomes. Chromosome Res 12: 767–776.
Mariguela TC, Roxo FF, Foresti F, Oliveira C . (2016). Phylogeny and biogeography of Triportheidae (Teleostei: Characiformes) based on molecular data. Mol Phylogenet Evol 96: 130–139.
Marquioni V, Bertollo LAC, Diniz D, Cioffi MB . (2013). Comparative chromosomal mapping in Triportheus fish species. Analysis of synteny between ribosomal genes. Micron 45: 129–135.
Martins C, Ferreira IA, Oliveira C, Foresti F, Galetti PM Jr . (2006). A tandemly repetitive centromeric DNA sequence of the fish Hoplias malabaricus (Characiformes: Erythrinidae) is derived from 5S rDNA. Genetica 127: 133–141.
Morielle E, Varella-Garcia M . (1988). Variability of nucleolus organizer regions in phyllostomid bats. Rev Bras Genet 11: 853–871.
Nanda I, Zend-Ajusch E, Shan Z, Grützner F, Schartl M, Burt DW et al. (2000). Conserved synteny between the chicken Z sex chromosome and human chromosome 9 includes the male regulatory gene DMRT1: a comparative (re)view on avian sex determination. Cytogenet Cell Genet 89: 67–78.
Nirchio M, Oliveira C, Ferreira IA, Granado A, Ron E . (2007). Extensive polymorphism and chromosomal characteristics of ribosomal DNA in the characid fish Triportheus venezuelensis (Characiformes, Characidae). Genet Mol Biol 30: 25–30.
O’Meally D, Patel HR, Stiglec R, Sarre SD, Georges A, Graves JAM et al. (2010). Non-homologous sex chromosomes of birds and snakes share repetitive sequences. Chromosome Res 18: 787–800.
Pazian MF, Shimabukuro-Dias CK, Pansonato-Alves JC, Oliveira C, Foresti F . (2013). Chromosome painting of Z and W sex chromosomes in Characidium (Characiformes, Crenuchidae). Genetica 141: 1–9.
Peichel CL, Nereng KS, Ohgi KA, Cole BLE, Colosimo PF, Buerkle AC et al. (2001). The genetic architecture of divergence between threespine stickleback species. Nature 414: 901–905.
Peichel CL, Ross JA, Matson CK, Dickson M, Grimwood J, Schmutz J et al. (2004). The master sex-determination locus in threespine sticklebacks is on a nascent Y chromosome. Curr Biol 14: 1416–1424.
Phillips RB, Konkol NR, Reed KM, Stein JD . (2001). Chromosome painting supports lack of homology among sex chromosomes in Oncorhynchus, Salmo and Salvelinus. Genetica 111: 119–123.
Ráb P, Rabova M, Pereira CS, Collares-Pereira MJ, Pelikanova S . (2008). Chromosome studies of European cyprinid fishes: interspecific homology of leuciscine cytotaxonomic marker the largest subtelocentric chromosome pair as revealed by cross-species painting. Chromosome Res 16: 863–873.
Reed KM, Phillips RB . (1997). Polymorphism of the nucleolus organizer region (NOR) on the putative sex chromosomes of Arctic char (Salvelinus alpinus) is not sex related. Chromosome Res 5: 221–227.
Rodrigues AS, Medrado AS, Diniz D, Oliveira C, Affonso PRAM . (2016). ZZ/ZW sex chromosome system in the endangered fish Lignobrycon myersi (Teleostei: Characiformes: Triportheidae). Comp Cytogenet 10: 245–254.
Rutkowska J, Lagisz M, Nakagawa S . (2012). The long and the short of avian W chromosomes: no evidence for gradual W shortening. Biol Lett 8: 636–638.
Sánchez S, Jorge LC . (1999). A new report of the ZZ/ZW sex chromosome system in the genus Triportheus (Pisces, Triportheinae). Cytologia 64: 395–40.
Scacchetti PC, Utsunomia R, Pansonato-Alves JC, Vicari MR, Artoni RF, Oliveira C et al. (2015). Chromosomal mapping of repetitive DNAs in Characidium (Teleostei, Characiformes): genomic organization and diversification of ZW sex chromosomes. Cytogenet Genome Res 146: 136–143.
Schmid M, Haaf T, Geile B, Sims S . (1983). Chromosome banding in Amphibia. VII. An unusual XY/XX-sex chromosome system in Gastrotheca riobambae (Anura, Hylidae). Chromosoma 88: 69–82.
Schmid M, Steinlein C, Yano CF, Cioffi MB . (2016). Hypermethylated chromosome regions in nine fish species with heteromorphic sex chromosomes. Cytogenet Genome Res 147: 169–178.
Shetty S, Griffin DK, Graves JAM . (1999). Comparative painting reveals strong chromosome homology over 80 million years of bird evolution. Chromosome Res 7: 289–295.
Sumner AT . (1972). A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res 75: 304–306.
Symonová R, Sember A, Majtánová Z, Ráb P . (2015). Characterization of fish genomes by GISH and CGH. In: Ozouf-Costaz C, Pisano E, Foresti F, Almeida Toledo LF (eds), Fish Cytogenetic Techniques. Ray-Fin Fishes and Chondrichthyans. CCR Press: Boca Raton. pp 118–131.
Telenius H, Carter NP, Bebb CE, NordenskjSld M, Ponder BAJ, Tunnacliffe A . (1992). Degenerate oligonucleotideprimed PCR: general amplification of target DNA by a single degenerate primer. Genomics 13: 718–725.
Traut W, Sahara K, Otto TD, Marec F . (1999). Molecular differentiation of sex chromosomes probed by comparative genomic hybridization. Chromosoma 108: 173–180.
Vítková M, Fuková I, Kubíčková S, Marec F . (2007). Molecular divergence of the W chromosomes in pyralid moths (Lepidoptera). Chromosome Res 15: 917–930.
Yang F, Trifonov V, Ng BL, Kosyakova N, Carter NP . (2009) Generation of paint probes by flow-sorted and microdissected chromosomes. In: Liehr T (ed), Fluorescence In Situ Hybridization (FISH) –Application Guide. Springer: Berlin. pp 35–52.
Yano CF, Bertollo LAC, Liehr T, Troy WP, Cioffi MB . (2016). W chromosome dynamics in Triportheus species (Characiformes, Triportheidae): an ongoing process narrated by repetitive sequences. J Hered 107: 342–348.
Yano CF, Bertollo LAC, Rebordinos L, Merlo MA, Portela-Bens S, Liehr T, Cioffi MB, (submitted). Evolutionary dynamics of rDNAs and U2 snDNAs in Triportheus (Characiformes, Triportheidae): high variability and particular syntenic organization.
Yano CF, Poltronieri J, Bertollo LAC, Artoni RF, Liehr T, Cioffi MB . (2014). Chromosomal mapping of repetitive DNAs in Triportheus trifurcatus (Characidae, Characiformes): insights into the differentiation of the Z and W chromosomes. PLoS One 9: e90946.
Yonenaga-Yassuda Y, Assis MFL, Kasahara S, L’Abbate M, Souza MJ . (1983). Nucleolar organizer regions in Akodon arviculoides (Cricetidae, Rodentia): evidence for the activity of rDNA genes in both X chromosomes of females. Cytogenet Cell Genet 35: 143–147.
Zwick MS, Hanson RE, McKnight TD, Islam-Faridi MH, Stelly DM, Wing RA et al. (1997). A rapid procedure for the isolation of C0t-1 DNA from plants. Genome 40: 138–142.
Acknowledgements
This study was supported by the Brazilian agencies CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico; Proc. No. 306896/2014-1), FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo; Proc. No. 2014/22532-7) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), Bolsista da Capes; Proc. No. BEX 11744/13-8). TE is partially supported by an Australian Research Council Future Fellowship (FT110100733).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Yano, C., Bertollo, L., Ezaz, T. et al. Highly conserved Z and molecularly diverged W chromosomes in the fish genus Triportheus (Characiformes, Triportheidae). Heredity 118, 276–283 (2017). https://doi.org/10.1038/hdy.2016.83
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hdy.2016.83
This article is cited by
-
Satellitome analysis illuminates the evolution of ZW sex chromosomes of Triportheidae fishes (Teleostei: Characiformes)
Chromosoma (2022)
-
Against the mainstream: exceptional evolutionary stability of ZW sex chromosomes across the fish families Triportheidae and Gasteropelecidae (Teleostei: Characiformes)
Chromosome Research (2021)
-
Evolution of the parthenogenetic rock lizard hybrid karyotype: Robertsonian translocation between two maternal chromosomes in Darevskia rostombekowi
Chromosoma (2020)
-
Chromosomal mapping of repetitive sequences in Hyphessobrycon eques (Characiformes, Characidae): a special case of the spreading of 5S rDNA clusters in a genome
Genetica (2020)
-
Emerging patterns of genome organization in Notopteridae species (Teleostei, Osteoglossiformes) as revealed by Zoo-FISH and Comparative Genomic Hybridization (CGH)
Scientific Reports (2019)