Abstract
There have been few attempts to synthesise the growing body of literature on phenotypic plasticity to reveal patterns and generalities about the extent and magnitude of plastic responses. Here, we conduct a review and meta-analysis of published literature on phenotypic plasticity in aquatic (marine and freshwater) gastropods, a common system for studying plasticity. We identified 96 studies, using pre-determined search terms, published between 1985 and November 2013. The literature was dominated by studies of predator-induced shell form, snail growth rates and life history parameters of a few model taxa, accounting for 67% of all studies reviewed. Meta-analyses indicated average plastic responses in shell thickness, shell shape, and growth and fecundity of freshwater species was at least three times larger than in marine species. Within marine gastropods, species with planktonic development had similar average plastic responses to species with benthic development. We discuss these findings in the context of the role of costs and limits of phenotypic plasticity and environmental heterogeneity as important constraints on the evolution of plasticity. We also consider potential publication biases and discuss areas for future research, indicating well-studied areas and important knowledge gaps.
Similar content being viewed by others
Introduction
Phenotypic plasticity is the environmentally contingent expression of phenotypes. Variation in the expression of phenotype from a single genotype can arise from two kinds of plasticity: developmental noise resulting from factors beyond the organism's genetic control and adaptive or regulated plasticity. Plasticity in phenotypic traits can therefore be adaptive (for example, modular or integrated modifications of growth, development and behaviour in response to environmental cues) or non-adaptive (for example, stressful environments or poor diets result in slow growth, low survival or low fecundity). Because genetic variation may underlie the expression and magnitude of phenotypic plasticity (Pigliucci et al., 2006), it can be acted on by natural selection and in some cases can become a powerful adaptation, allowing organisms to better match their phenotype to prevailing environmental conditions and to maintain their function in a variable environment. Furthermore, plasticity may influence species interactions, promote the origin of novel phenotypes, divergence among populations and species, and adaptive radiation (West-Eberhard, 1989, 2005; Agrawal, 2001; Pigliucci et al., 2006; Pfennig et al., 2010).
An extensive theoretical literature has identified conditions under which plasticity should be favoured by natural selection over fixed phenotypes. In general, plasticity should evolve when the fitness of individuals with plastic phenotypes is greater than that of individuals with fixed phenotypes. This requires (i) spatial and/or temporal environmental heterogeneity, (ii) reliable environmental inducing cues, (iii) fitness benefits that outweigh the fitness costs of plasticity and (iv) a genetic basis for plasticity (for reviews, see Tollrian and Harvell, 1999 and Berrigan and Scheiner, 2004, and references therein).
Research during the last several decades has shown that aquatic (marine and freshwater) gastropods can alter shell form adaptively during ontogeny in response to effluent from their predators. These plastic responses appear ubiquitous; they have been found in multiple species (for example, Appleton and Palmer, 1988; Palmer, 1990; Trussell, 1996; DeWitt, 1998; Hoverman et al., 2005; Hollander et al., 2006; Bourdeau, 2009; Brönmark et al., 2011), in species with different dispersal capabilities (for example, Behrens Yamada, 1989; Hollander et al., 2006), from different geographic locations (for example, Trussell, 2000a, b; Trussell and Smith, 2000) and in response to several predator species (for example, Hoverman and Relyea, 2007; Bourdeau, 2009). However, populations and species often differ in their degree of shell plasticity (for example, Appleton and Palmer, 1988; Palmer, 1990; Trussell, 1996; Edgell and Neufeld, 2008; Edgell et al., 2009; Bourdeau, 2012). Environmental factors other than predator cues can also modify or limit the plasticity of gastropod shell form (for example, water motion (Hollander et al., 2006; Minton et al., 2007, 2011; Dillon et al., 2013), temperature (Melatunan et al., 2013) and calcium availability (Rundle et al., 2004)). In the case of water temperature, thinner shells are predicted to develop in relatively cold water because the amount of calcium carbonate needed to saturate cold water is larger than that in warm water and dissolution rates increase with decreasing water temperature. However, shell thickness may be higher in cold water than in warm water because gastropods grow more slowly in cold temperatures, indicating a temperature effect separate from the calcium carbonate effect (Vermeij, 1982a, b).
Despite becoming a model system to study phenotypic plasticity (for example, DeWitt, 1998; Auld and Relyea, 2011; Brönmark et al., 2011), there have been no attempts to synthesise the available literature on phenotypic plasticity in aquatic gastropods to reveal (i) patterns and generalities about the extent and magnitude of phenotypic plasticity, and (ii) research topics that have been widely addressed across systems versus others that have not been studied and so represent substantial knowledge gaps.
Understanding the environmental conditions influencing the extent and magnitude of plastic phenotypic responses is important for several reasons. Phenotypic plasticity can reflect an adaptive evolutionary response to spatial and temporal variation in species interactions, which can influence the structure and function of food webs (Turner and Mittelbach, 1990; Chase, 1999; Trussell et al., 2002; Peacor and Werner, 1997). Moreover, the expression of phenotypic plasticity by one species can lead to reciprocal phenotypically plastic change in other interacting species (Kopp and Tollrian, 2003; Kishida et al., 2006; Edgell and Rochette, 2009). Phenotypic plasticity may also facilitate adaptation to novel environments (Yeh and Price, 2004; Richards et al., 2006; Ghalambor et al., 2007), and contribute to genetic differentiation and speciation (West-Eberhard, 1989; Price et al., 2003; Pfennig et al., 2010). Further, because costs and limits are likely to constrain the evolution of adaptive phenotypic plasticity (DeWitt et al., 1998; Auld et al., 2009), documenting the environments and taxa in which adaptive phenotypic plasticity evolves can provide information about conditions in which the benefits of plasticity outweigh the costs and which limitations constrain the adaptive value of phenotypic plasticity.
In this study, we conducted a meta-analysis to synthesise the published literature on aquatic gastropod phenotypic plasticity. The available data allowed us to make two comparisons of phenotypic plasticity for shell, life history and growth traits: (i) between marine and freshwater species; and (ii) between marine species with planktonic versus crawl away dispersal. We discuss possible drivers of the patterns we observed, and where more data are needed to test causal hypotheses.
Materials and Methods
Literature search
We conducted a literature search using the ISI Web of Science (WoS) database and search engine. WoS does not include all scientific journals and does not search all books and monographs, but it does search a broad range of primary literature and provides repeatable results without introducing bias by the researcher. To identify as much of the available and relevant literature as possible, we used the following search string (that is, using keywords): Topic=(snail* OR gastropod*) AND (plastic* OR phenotyp* plastic* OR induc*) AND (marine OR freshwater OR aquatic). We only searched English language publications. The initial search included records from 1900 to November 2013 and identified 944 studies. Next, we limited our database to relevant fields of study by using the ‘refine’ function in WoS to exclude non-relevant subjects such as: medicine, agriculture, engineering and physics. We further used the ‘refine’ function to limit our database by document type, excluding reviews, meeting abstracts, corrections and book chapters. This resulted in 562 total articles.
We examined the titles and abstracts of these 562 studies to determine whether they focused on examining plastic responses of gastropod phenotype (for example, behaviour, morphology, life history) to one or more environmental selective agents (for example, predators, abiotic factors). We excluded studies that did not meet these criteria; close to 400 articles were rejected because they were not related to the topic of gastropod phenotypic plasticity (for example, they were not about phenotypic plasticity or concerned plastics in the environment) or they were excluded for other reasons (for example, were not studies on gastropods). This left us with 176 studies, the first of which was published in 1985. Only a small number of studies matched our search criteria prior to 2004, with fewer than five studies per year, on average, from 1985 to 2004 (Figure 1). This number more than doubled by 2007 to approximately 14 studies per year between 2007 and 2013.
The number of studies published per year included in the initial search prior to the systematic review (n=176 studies). The most recent year (2013) only included records in the database through November (journals published at different dates in November will vary in their inclusion in the database) and indexed on the Web of Science as of November 2013.
We further evaluated these 176 studies by examining the full text of each paper. After full text evaluation, 80 studies were excluded for a variety of reasons; 15 studies did not experimentally test for plasticity, 17 were strictly descriptive or observational, 11 used only genetics or molecular data to infer plasticity, 8 were synthetic studies with no original data, 4 were modeling studies, 3 were taxonomic comparisons, 2 were concerned with inducible trophic structures (that is, ‘inducible offenses’), 2 were studies of the functional performance of previously induced phenotypes, 2 were concerned with learning plasticity, 2 were not studies of gastropods, 1 was a paleontological study and 3 were excluded for other reasons. Once initial decisions were made, all studies (both marine and freshwater) were checked and discussed, and the list was finalised. In addition to our literature exclusion process, we also searched the references in selected studies for additional literature that was missed through keyword search. Our final tally of studies for the systematic review was 96.
We developed a database for these studies using EndNote Web (https://www.myendnoteweb.com/EndNoteWeb.html), importing initial results from WoS. We then developed an MS Excel spreadsheet for entering the data we collected from each source. The data are available in Supplementary material.
Systematic review
Studies included in the systematic review were categorised first by ecosystem type (marine or freshwater) and the region where they were carried out (temperate, sub-tropical (including Mediterranean climates) and tropical). We also categorised studies by type of study, including experimental laboratory or mesocosm studies, experimental field studies or a combination of laboratory or mesocosm experiments and field observations or experiments. We then identified the focal species (that is, the species exhibiting phenotypic plasticity) to family, genus and species. If there was more than one species in a study, we treated each one independently. In addition to taxonomic categorisation, we also categorised the focal species by dispersal type (planktonic or crawl-away young). This latter categorisation was included to test whether dispersal ability had an effect on the magnitude of plasticity.
The environmental factor that was manipulated as the inducing agent in each study was characterised as predator, abiotic factor or habitat. The habitat category was applied only to studies where focal species were transplanted between different habitats, but where habitats were not specifically defined by a particular inducing factor (for example, predators). Thus, if a field transplant occurred between habitats defined specifically by differences in predator abundance, the inducing agent was characterised as ‘predator’. If the inducing agent was a predator, it was characterised as a generalist or specialist (with respect to gastropod prey), whether it was one of multiple predators or in isolation, whether it was native or invasive to the focal prey’s native habitat, and whether the predator was presented to the prey with or without damaged conspecific prey. For each study, the type of trait that exhibited plasticity in response to the inducing agent was categorised as: shell morphology, growth/life history (this included traits like absolute growth, growth rate and time to maturity or first reproduction), behaviour, physiology or soft-tissue morphology. Note that, whereas studies that focused solely on behavioural plasticity were excluded from our synthesis, if behavioural plasticity was measured in addition to shell plasticity within a study, we noted it and characterised it. If multiple plastic traits were measured, we categorised each one independently.
Meta-analysis
We used meta-analysis to quantify variation in magnitude of plastic responses to environmental factors (that is, inducing agents) in the studies from our systematic review and to elucidate patterns detailed above. Meta-analysis is a statistical tool used to synthesise multiple, independent data sets into a single data set used to test predictions. Here, we used the same method for meta-analysis as in Hollander (2008). As a measure of the magnitude of plastic responses we quantified an effect size for each study using Hedge’s d (Gurevitch and Hedges, 1993). The effect size was calculated from the standardised difference between means from the experimental treatments and control groups.
We calculated the absolute difference in trait means between inducing and non-inducing (control) environments rather than the difference in any one direction for two reasons. First, an adaptive trait change in a given environment could require either an increase or a decrease in trait value. For some environmental changes, there might even be multiple adaptive response strategies such that a shift in some traits may potentially be adaptive in either direction. Second, our comparisons between habitats and dispersal modes are focused on the relative magnitude of plastic responses. As such, it is essential to compare the non-directional difference in the phenotype between inducing and non-inducing environments. Although the magnitude of phenotypic responses can depend on the strength of the inducing environmental cue, there is no standardised way to measure cue strength among studies. Consequently, we did not make any adjustment to our estimate of plastic response magnitude based on cue strength. We assume that inducing cues or environments should not differ systematically between marine and freshwater environments and that inducing cues used in experiments were representative of the environments experienced by the study species.
Each study was weighted by its sample size and a standardised measure of variation. Because we assumed a random component of variation among effect sizes (Rosenthal et al., 2000), we used a mixed model together with bias-corrected 95% bootstrap confidence intervals (Adams et al., 1997). The analyses were conducted in the statistical software MetaWin 2.0 (Rosenberg et al., 1997). Furthermore, we confirmed that the direction of the effect between experimental treatments and the control was always positive (Gurevitch et al., 1992). We verified the robustness of data with respect to publication bias; when non-significant results have a lower publication rate compared with significant results (designated as the ‘file-drawer effect’; Rosenthal, 1984). We examined a funnel graph, suggested by Palmer (1999), and conducted a fail-safe sample size analysis to test how many studies with zero effect would be needed to reject our hypotheses (Rosenthal, 1979). Both the funnel graph and Rosenthal’s method suggested a robust data set where publication bias was unlikely. To test for effects of non-independence between traits or species within studies, or between-studies for a given species, we reduced the number of traits within each study and used only one study from each species; all to condense the analyses of our dataset. This procedure confirmed our conclusions although with larger confidence intervals due to reduced statistical power.
Results
Systematic review
Plasticity experiments on aquatic gastropods have largely been laboratory experiments in the absence of any complementary field observations or manipulations (48% of the studies; n=46 of 96), followed by laboratory experiments combined with field observations or manipulations (36% of studies; n=35) and isolated field manipulations (16% of studies; n= 15). Marine taxa were the focus of 72% of these studies, with freshwater taxa being the focus of the remaining 28%. Most studies were conducted in temperate climates (85% of marine studies, 96% of freshwater studies).
Both marine and freshwater studies were dominated by a narrow subset of gastropod taxa. Eighty-one percent of marine studies involved two families; Littorinidae (56%) and Muricidae (25%). No other family accounted for more than 5% of the studies in marine systems. Forty percent of freshwater studies were on snails in the family Physidae, with snails in the families Lymnaeidae and Planorbidae each representing an additional 27% of the studies (Figure 2).
Marine studies have included taxa with different reproductive and dispersal modes (60% oviparous with crawl-away young, 29% with planktonic development, 11% on a single ovoviviparous species with crawl-away young (Littorina saxatilis)), whereas freshwater studies have only been carried out on oviparous taxa with crawl-away offspring.
Studies of shell morphology and/or snail growth and life history traits dominated the literature (Figure 3a). Whereas marine studies have focused mainly on shell morphology (50% of studies), followed by growth/life history (40%), freshwater systems have followed the reverse order (53% growth/life history, 44% shell morphology). In both marine and freshwater systems, all other traits were measured in fewer than 5% of the total studies (Figure 3a). Multiple plastic traits and/or correlations between multiple plastic traits (that is, trait integration) were studied in 20% of all freshwater studies and 15% of all marine studies.
Predator-induced plasticity dominated the literature for both marine (62% of studies) and freshwater systems (78%). Of these studies, generalist predators (shell-breaking crabs and aperture-entering sea stars) were used exclusively in marine systems, whereas shell-breaking gastropod specialists (43%), shell-breaking and shell-entering generalist predators (26%), and a combination of both (30%) were used in freshwater systems. Predators were most commonly native with respect to the prey (55% of studies), and rarely invasive (9% of studies); about one-third of studies incorporated both native and invasive predators (35% of studies).
Habitat type, which often differed in multiple environmental factors (for example, wave-exposed versus wave-protected habitat in marine environments, or eutrophic versus oligotrophic in freshwater environments), was the next most common inducing factor (20% of all marine studies and 11% of freshwater studies). Abiotic factors (for example, water motion or temperature) were the third most common type of study (13% of marine studies and 7% of freshwater studies). Studies that combined predators and abiotic factors accounted for fewer than 5% of studies in both habitat types (Figure 3b).
Results of the meta-analysis
The meta-analysis included studies from 1985 to November 2013. These studies included 48 marine studies covering nine families (Littorinidae, Muricidae, Patellidae, Trochidae, Buccinidae, Cypraeidae, Columbellidae, Potamididae, Turbinidae) and 17 freshwater studies with representatives from five families (Physidae, Lymnaeidae, Planorbidae, Hydrobiidae, Pleuroceridae). This allowed us to compare the magnitude of plastic responses between marine and freshwater taxa and test whether those magnitudes differed. Further, within marine taxa, there were 16 studies on taxa with planktonic dispersal and 32 on taxa with crawl-away young, enabling us to compare the magnitude of plastic responses between taxa with different dispersal capacity.
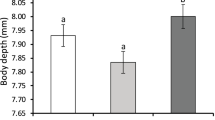
For both marine and freshwater gastropods, mean effect sizes for shell thickness, shell shape and life history were moderate to large and had confidence intervals that did not overlap zero (Figure 4) indicating strong and significant plastic responses. Freshwater gastropods exhibited greater plasticity for shell thickness (Qbtw(1,60)= 37.69, P<0.001; Figure 4a) and shell shape (Qbtw(1140)= 83.70, P<0.001; Figure 4b) compared with marine gastropods. The difference in plastic shell shape responses between marine and freshwater taxa (Figure 4b) was twice as large as the difference in plastic shell thickness responses between marine and freshwater taxa (Figure 4a). Patterns of plasticity for growth and life history traits paralleled patterns in shell plasticity, with freshwater snails exhibiting significantly greater plasticity than their marine counterparts (Qbtw(1193)= 176.87, P<0.001; Figure 4c).
Within marine taxa, plastic responses were not greater in species with higher dispersal rates (planktonic development) compared with those with lower dispersal rates (crawl-away young) (Qbtw(1232)= 0.021, P=0.903; Figure 5).
Discussion
Our systematic review and meta-analysis identified several generalisations. Studies on aquatic gastropod phenotypic plasticity were extremely uncommon until the mid-1990s (Figure 1). Since that time, the number of studies per year has doubled and the majority of studies have been based on laboratory experiments in isolation or in combination with field observations. The early work of AP Covich (for example, Crowl and Covich, 1990), AR Palmer (for example, Appleton and Palmer, 1988; Palmer, 1990), and GJ Vermeij (for example, Vermeij, 1974, 1978) strongly influenced the field by focusing attention on predator-induced plasticity in shell morphology and life history of a few key taxa. To date, studies in marine systems have outpaced those in freshwater systems. In both systems, the literature is dominated by a few, highly studied genera and disproportionately from temperate rather than tropical latitudes. Here, we first discuss the results of the meta-analysis and then address the findings of the literature review.
Meta-analysis and the marine-freshwater comparison
We found, on average, that freshwater gastropods exhibit three times greater plastic responses in phenotype than marine taxa (Figures 4a and c). Further, within marine taxa, we found no difference in plastic response between gastropods with planktonic and benthic development.
It is interesting that we found larger plastic shell responses in freshwater gastropods (relative to marine species), and it is tempting to attribute this pattern to differences in the calcium availability between these habitats. For example, if the costs of accumulating, transporting and precipitating CaCO3 are greater when calcium is limited (for example, in freshwater systems), then our findings would fit the prediction that stronger plastic responses should evolve when constitutive production of a phenotype is relatively costly (see Tollrian and Harvell, 1999 and Berrigan and Scheiner, 2004 and references therein for reviews of theoretical predictions). However, at present, it is not clear whether the cost of calcification is indeed greater in freshwater gastropods compared with marine taxa (Palmer, 1992).
Depending on the relationship between calcium availability and the cost of calcification, one could hypothesise that calcium limitation could lead to either greater or lesser plastic shell responses in aquatic gastropods. On one hand, calcium limitation could constrain high baseline levels of calcium carbonate deposition and relegate allocation of additional shell deposition (for example, to be used for shell thickening) to instances when predators are detected, leading to relatively large plastic responses in freshwater taxa. Likewise, in marine habitats where calcium is more readily available, gastropods may be able to more easily deposit greater baseline levels of calcium carbonate (Palmer, 1992), leading to more heavily calcified (for example, thicker) constitutive shell morphology; a consequence of which might be less scope or necessity for inducible shell thickening responses in marine snails. On the other hand, limited calcium availability could constrain both constitutive and inducible levels of shell deposition. Indeed, experimental reduction of calcium availability has been shown to limit plastic shell-thickening responses in both marine and freshwater gastropods (Rundle et al., 2004; Bibby et al., 2007; Bukowski and Auld, 2014).
Plastic shell responses are not limited to shell thickening via increased calcium carbonate deposition, and both marine and freshwater gastropods are capable of plastically altering different aspects of their shell shape (for example, aspect ratio; DeWitt, 1998; Hollander et al., 2006; Bourdeau, 2009). Altering shell shape may not require additional investment in shell deposition, just re-allocation of shell material to different shell locations, which could allow for the development of adaptive shell forms without the necessity for increased shell deposition in calcium-limited habitats. Indeed, it has been suggested that freshwater snails should be more likely than marine taxa to alter their gross shell shape (for example, aspect ratio) in response to predators rather than increase their shell thickness, because calcium carbonate availability is limited in freshwater systems (for example, DeWitt, 1998). Thus, the larger plastic responses in shell shape in freshwater compared with marine snails may reflect an evolutionary strategy for dealing with environmental constraints on shell deposition in calcium-limited freshwater.
Before drawing robust conclusions about the role of calcium limitation and costs of calcification driving patterns of phenotypic plasticity in gastropod shell form, we need a better understanding of how gastropods manage the costs of different shell shapes and thicknesses. For example, an increase in whorl expansion rate allows for the conservation of calcium carbonate, by decreasing the surface area to volume ratio of the shell (Raup 1961). As such, evolution can favour developmentally fixed patterns of increased whorl expansion rate in place of phenotypic plasticity to conserve the deposition of calcium carbonate in gastropods. Further, marine and freshwater gastropods could have very different calcium carbonate concentration thresholds for shell deposition and dissolution (Clarke, 1978) and so any assumptions about magnitudes of costs or constraints is unwarranted without additional data. Further study will therefore be needed to understand the precise mechanisms by which aquatic gastropods have overcome calcium carbonate limitations. We propose that particularly illuminating studies would test whether gradients of calcium-limitation cause population-level differences in the expression of shell whorl expansion and plastic shell deposition within species of both marine and freshwater taxa.
Another important factor to consider is the organic fraction of the gastropod shell. Although gastropod shell is mainly composed of calcium carbonate, there is a small organic fraction; the production of which is more expensive than calcium carbonate deposition (Palmer, 1983), and may limit the production of well-defended shells (Palmer, 1992). Thus, the metabolic process of plastically altering shell form can be limited by both calcium availability (Rundle et al., 2004) and the cost of producing organic material (Palmer, 1992). Consistent with the idea that organic shell material is costly to produce, recent experimental evidence suggests that predator-induced thickening of shells in marine gastropods is caused by the deposition of mechanically weak and energetically inexpensive material with low organic content (Bourdeau, 2010). Understanding how the environment influences the ability of gastropods to produce the organic shell fraction, which at least for marine gastropods is more expensive than the calcium carbonate deposition (Palmer, 1983), will be critical for understanding the patterns of variation in the nature and magnitude of shell plasticity in aquatic gastropods.
Environmental variability of marine versus freshwater environments
An alternative hypothesis to explain our findings is that greater plasticity in freshwater gastropods is due to greater environmental heterogeneity in the shallow ponds and streams from which most of the experimental freshwater snails in the studies came, compared with the rocky intertidal habitats from which the majority of the experimental marine snails came. However, we do not have data to directly assess this hypothesis. Shallow ponds and streams are extremely variable in ways that may incur large within- and between-generation variation in predation risk for gastropods (for example, spatial and temporal variation in pond permanence, seasonal and year-to-year variation in flow regime in stream systems), but so too are rocky intertidal habitats in nearshore coastal systems. In some cases, our understanding of spatial and temporal environmental variation in the intertidal zone is relatively sound. For example, tides are predictable and so approximate emersion times can be calculated for gastropods at any height on the shore (for example, Denny and Paine, 1998). However, although the temporal pattern of the tides is well understood, much of our current understanding of the spatial and temporal patterns of other environmental variation in the intertidal zone is inadequate to evaluate their evolutionary role in driving phenotypic plasticity of marine gastropods. For example, multiple environmental factors, such as the availability of resources and refugia, the abundance and diversity of predators, and conspecific density will all contribute to the environmental heterogeneity selecting for the nature and magnitude of phenotypic plasticity in aquatic gastropods.
Further complicating matters, an organism's dispersal capacity will also determine, in part, the extent of environmental heterogeneity an organism experiences (Via and Lande, 1985; Brown, 1990; Hollander, 2008). Dispersal modes in marine gastropods can be categorised into two broad strategies: (1) viviparous/ovoviviparous development or direct development from benthic egg masses and (2) planktonic larval dispersal with a benthic adult stage. For species exhibiting strategy 1, theory predicts a relatively fixed, locally adapted genotype because individuals do not disperse great distances, that is, gene flow is limited, and offspring environment is predictably similar to parental environment. In contrast, theory predicts that species exhibiting strategy 2 should evolve phenotypic plasticity because dispersal leads to exposure to a broad range of potential environments, which are unpredictable on the basis of parental environment. However, the capacity for dispersal may not reflect how the organism experiences environmental variability. For example, freshwater gastropods may lack a planktonic stage, but they can be dispersed as juveniles or adults by water birds and mammals (Wesselingh et al., 1999, Figuerola and Green, 2002, Van Leeuwen, 2012; Van Leeuwen et al., 2013). Further, marine species with benthic development may disperse widely via rafting on seaweeds (Highsmith, 1985; Martel and Chia, 1991; Donald et al., 2011). It is becoming increasingly clear that direct developing species with low dispersal capacity can display strong plastic responses, whereas planktonic developers with high dispersal capacity may exhibit little or no plasticity (Behrens Yamada, 1989; Parsons, 1997; Hollander et al., 2006). Our meta-analyses support these findings and suggest that larval dispersal may not have a large role in creating the environmental heterogeneity marine gastropods experience once they settle, nor in selecting for plasticity. More work is needed on how dispersal potential affects actual dispersal distances and habitat encounter rates, which will define how aquatic gastropods experience their environment.
Prevalence of phenotypic plasticity in aquatic gastropods and potential biases
Our review clearly indicates that phenotypic plasticity is widespread in aquatic gastropods (Figure 1). However, studies have been primarily focused on a few well-studied genera known to exhibit plasticity, and this can bias perceptions of the prevalence of plasticity. Note, however, that Edgell and Hollander (2011) showed that plasticity is indeed prevalent among marine invertebrates, including many gastropods. Nevertheless, taxa that are known to display plasticity are both more likely to be studied and to be published, possibly exaggerating the importance and magnitude of phenotypic plasticity in aquatic gastropods. We need more studies on species where plasticity is not expected or expected to vary in magnitude among species (for example, Hollander et al., 2006; Bourdeau, 2011; Edgell and Hollander, 2011) and more reports of studies where plasticity is tested for but not found (for example, Pernet, 2007), to be sure that the magnitude and importance of plastic responses in aquatic gastropods is not overstated.
Indeed, even among closely related gastropod species within a genus, not all species will respond plastically to the presence of predators (for example, Hoverman et al., 2014). There is a strong tradition of inter-population comparisons of phenotypic plasticity in the aquatic gastropod literature (for example, DeWitt, 1998; Edgell et al., 2009 and Brönmark et al., 2011), which has provided a great deal of evidence for intraspecific variation in levels of shell plasticity. Interspecific variation in levels of plastic response may also be expected, but such comparisons are rare (but see Langerhans and DeWitt, 2002; Hollander et al., 2006; Bourdeau, 2011; Hoverman et al., 2014). Indeed, there are ecological conditions that will favour canalised or fixed phenotypic development over plastic strategies (Levins, 1968). For example, we may expect that constitutive shell defenses should be favoured over plastic responses in the tropics due to high and predictable encounter rates with predators (Poitrineau et al., 2004), and lower costs associated with calcification (Palmer, 1992). An ideal research programme would focus on groups with well-resolved phylogenies and with many species that are amenable to direct experimentation, but predicted to be more or less phenotypically plastic. Such studies will reveal how widespread and common plastic responses are, while simultaneously allowing us to address fundamental questions about the evolutionary causes and ecological consequences of plasticity. Such studies have been carried out in amphibians (another taxon known for morphological plasticity) with illuminating results. For example, in a comparison of predator-induced plastic responses in 16 species of larval amphibians, Van Buskirk (2002) found a significant, phylogenetically independent correlation between morphological plasticity and predator variability, supporting the hypothesis that high levels of plasticity will evolve in highly variable environments. Similar studies could also be implemented to address the question of whether phenotypic plasticity affects speciation or diversification rates (Pfennig et al., 2010).
Another potential bias in our study is that almost all of the marine studies are based on hard-bottom, intertidal gastropods. Soft-bottom taxa are mostly absent (but see Martín-Mora et al., 1995 and Delgado et al., 2002 for examples of a species found on both rocky and soft-bottom substrates), as are studies involving predators that use methods other than shell breakage (but see Edgell et al., 2009 and Bourdeau, 2009 for exceptions). It is possible that gastropods living in soft-sediment habitats or those preyed on mainly by slow-moving shell-entry predators (for example, seastars) may rely chiefly on plastic avoidance and/or escape behaviour as their antipredator defense, rather than plastic morphological defenses. More studies of soft-bottom taxa and studies using shell-entry predators as inducing agents will prove valuable for testing the importance and generality of plastic shell defenses in marine taxa.
Types of studies
Phenotypic plasticity in aquatic gastropods has thus far been studied intensively in experimental settings in the laboratory and in mesocosms, but much less frequently in nature. Therefore, the relevance of laboratory findings to the real world is poorly known. We note that observations of phenotypic variation in nature, which often preceded or accompanied laboratory induction experiments, are a critical component to studies of plasticity because they suggest the appropriate traits to examine and environmental factors to manipulate in subsequent experiments. Our review indicates that studies of gastropod plasticity have a strong tradition of being motivated by observations of phenotypic differences between habitats or among spatially separated populations (see for example, Appleton and Palmer, 1988 and Trussell and Smith, 2000). In many of these cases, field observations have identified the variable traits (for example, shell thickness and spire height) and habitat characteristics (for example, crab-free, wave exposed habitats versus crab-rich, wave protected habitats in marine systems) necessary to conduct meaningful laboratory experiments. However, not all research has emerged in response to observations of naturally occurring patterns and we caution that the absence of good field data can result in an experimental focus that does not match the patterns in need of explanation.
Whereas the focus on induction studies in laboratory or mesocosm settings has provided valuable information about which environmental factors can induce plastic responses, and how gastropods respond to those factors, it does not tell us whether or to what extent plastic responses are being induced in nature; nor does it tell us whether the induced responses provide an adaptive advantage under natural conditions. Only 15 of the 96 studies in the survey include direct field manipulations and even fewer showed directly that the induced phenotypes were adaptive. More field experiments are needed, as they have the potential to help determine whether the responses observed in laboratory experiments are relevant in natural settings. In one prominent example where the relevance of laboratory-induced traits to field performance was tested, Hollander and Butlin (2010) showed that relatively small plastic responses induced in the laboratory in Littorina saxatilis had significant impacts on snail fitness in the field. Near-shore benthic marine gastropods and shallow water freshwater gastropods provide an ideal opportunity for such studies as they are highly amenable to field manipulations. Further, the emerging body of literature describing laboratory experiments in aquatic gastropods provides clear predictions about how natural populations should differ if phenotypic plasticity is operating. We therefore call for future studies to incorporate field manipulations when feasible, as they will allow researchers to assess whether phenotypic variation observed in the field and plastic variation induced in laboratory experiments are consistent, which will facilitate interpretation about whether induction mechanisms known from experiments are occurring in nature, and provide strong tests for the adaptive consequences of plastic responses (for example, Hollander and Butlin, 2010). We note, however, that such manipulations should be carried out with extreme care, because if transplanted animals escape their enclosures, the experiment risks gene transfer that could potentially erode the evolutionary divergence underlying the predicted geographic and spatial differences in phenotypic plasticity.
Biogeographic patterns of phenotypic plasticity
At present, we are unable to address the question of whether shell plasticity is more prevalent in temperate or tropical gastropods, or whether the magnitude of shell plasticity differs among these zones, because the literature is dominated by studies focused on marine gastropods in temperate systems. Temperate marine habitats generally exhibit greater variability in physical environmental factors (for example, temperature, waves, storms; Sobel, 2012) as well as predation pressure (Vermeij, 1978) than tropical habitats (Bertness et al., 1981). Such variability may favour increased phenotypic plasticity (Levins, 1968, reviewed in Berrigan and Scheiner, 2004), and so we might expect to find more plastic phenotypes in response to such environmental fluctuations. Calcium carbonate is also more bio-available in warm tropical waters than cold temperate waters, which may also contribute to higher plasticity at high latitudes if calcification of shells entails a greater energetic cost. We are aware of only one tropical species in which shell plasticity has been demonstrated. The queen conch (Strombus gigas) shows marked developmental plasticity in shell shape (Martín-Mora et al., 1995) and predator-induced plasticity in behaviour, growth and shell thickness (Delgado et al., 2002). We need many more studies on tropical marine and freshwater gastropods, as it would be interesting to know whether shell plasticity is a general phenomenon in tropical gastropods and to test whether patterns of variation in shell plasticity in temperate and tropical aquatic snails reflect differences in environmental variation between temperate and tropical zones.
Types of traits studied
Demographic and life history characteristics, including absolute growth, growth rates, size and age at maturity and fecundity, were often included in studies of gastropod shell plasticity. Gastropods often face a tradeoff between somatic growth and shell development. This tradeoff can arise either as a direct energetic or developmental tradeoff (Appleton and Palmer, 1988; Palmer, 1990) or can be mediated through altered feeding activity (Bourdeau, 2010). Thus, it is not surprising that these parameters are included in studies. What is perhaps more surprising is that fitness costs, whether in the form of reduced somatic growth, fecundity or survivorship, are not explicitly quantified or accounted for more often. For example, correlations between the expression of plastic shell responses and fitness-related costs (for example, reductions in feeding, somatic growth or reproductive output) were explicitly quantified in only five freshwater studies and four marine studies. Instead, costs are often assumed on the basis of previously observed negative correlations between shell development and growth and/or fecundity in other species. While in some cases, such assumptions of costs are likely to be sound, careful consideration of the causal mechanisms linking the expression of the plastic phenotype and its effects on fitness-related traits across a range of environments will be required to draw strong inferences about costs associated with plastic shell expression; such approaches are rare in studies of aquatic gastropods (but see DeWitt, 1998 and Brönmark et al., 2011 for notable exceptions).
Adaptive value of phenotypic plasticity
Rarer still are studies that have directly tested the adaptive value of the plastic shell responses of gastropods following their induction. In marine gastropods, it is assumed that induced shell thickening decreases vulnerability to the attack of shell-breaking crabs. However, in one of the few studies that tested this assumption, Edgell and Neufeld (2008) found that crab-induced thickening at the shell lip provided no survival advantage to Nucella lamellosa when crab predators attacked the relatively thin-walled spire. Furthermore, the induction of a defended phenotype may affect the prey organism’s performance in ways other than its susceptibility to attack by the inducing predator. Studies have shown that a phenotype induced by one predator may make gastropod prey more vulnerable to attack by other predators (DeWitt et al., 2000; Bourdeau, 2009; Hoverman and Relyea, 2009). Thus, the full consequences of induced phenotypes are only revealed when performance in multiple environments is examined. Future studies examining the costs and benefits of an induced phenotype across multiple environmental contexts should provide a more complete picture of the ecological consequences of plastic responses. Further, to our knowledge, there are not any studies that have examined whether predator-induced plasticity for traits that may reduce detection by predators (for example, shell withdrawal depth) provide the gastropod with any advantage at the detection stage of predation.
Explicit consideration of patterns of functional, developmental and/or genetic associations among different traits has been promoted as a way to gain a more complete understanding of constraints on phenotypically plastic responses and the costs and benefits of alternative phenotypes (Pigliucci, 2003, DeWitt and Scheiner, 2004). Aquatic gastropods have provided some of the best examples of explicit consideration of functional correlations among plastic traits and so-called ‘trait integration’ (for example, DeWitt, 1998; DeWitt et al., 1999; Rundle and Brönmark, 2001; Cotton et al., 2004; Hoverman et al., 2005; Brookes and Rochette, 2007; Bourdeau, 2010). Despite this, our review revealed that other types of plastic traits (for example, behavioural, physiological and so on) were rarely included in studies of gastropod shell plasticity, and when they were functional, mechanistic or genetic correlations between them were often not analysed (but see DeWitt, 1998; DeWitt et al., 1999; Langerhans and DeWitt, 2002; Hoverman et al., 2005; Brookes and Rochette, 2007; Bourdeau, 2010; Brönmark et al., 2011). We suggest that future studies of aquatic gastropod shell plasticity should incorporate measurements of multiple morphological, physiological and behavioural traits to clarify the link between plastic responses and phenotypic expression and their associated costs.
Studies of phenotypic plasticity do not often attempt to distinguish between adaptive and non-adaptive aspects. However, both adaptive and non-adaptive plasticity can be informative as they both result from interesting biological mechanisms. Adaptive plasticity often appears to fit functional predictions, whereas non-adaptive plasticity might reflect underlying genetic and developmental constraints on phenotype development. For example, many of the studies of predator-induced shell thickness, in marine snails in particular, document a correlated reduction in snail feeding and soft tissue growth. In some cases, crab-induced shell thickening is due to increased deposition of calcium carbonate (Appleton and Palmer, 1988; Palmer, 1990; Brookes and Rochette, 2007), which is presumed to be traded off against investment in soft tissue growth. However, in other cases, shell thickening has been shown to be an inevitable consequence of reduced snail growth because calcium carbonate is deposited at a constant rate regardless of snail growth, and reduction in linear shell growth results in shell accretion (and thus thickening) at the apertural lip (Bourdeau, 2010). Shell shape variation in western Atlantic Littorina littorea is also a function of snail growth rate (Kemp and Bertness, 1984). Rapid growth results in a thin-shelled globose shell morph composed of a reduced proportion of calcium carbonate, in comparison with slower growing snails; a result of a fixed rate of calcium carbonate deposition. If gastropods have only limited control over the rate of calcium carbonate deposition, the implication is that shell form plasticity is closely tied to growth rate and that plastic responses in shell form may simply be a consequence of changes in any environmental condition (predator or otherwise) that slows snail growth rather than an evolved adaptive and regulated predator-induced response. More studies aimed at examining the causal link between growth rate and shell form will aid in distinguishing between these alternative explanations for the evolution of shell form plasticity in aquatic gastropods (Vermeij, 2005).
Predator-induced plasticity versus abiotically induced plasticity
Predator-induced plasticity dominated the studies of plasticity in both marine and freshwater gastropods. Studies examining the plastic responses to other biotic and abiotic inducing agents on gastropod shells were much less common. This finding is in contrast to a taxonomically broader systematic review of phenotypic plasticity in marine invertebrates and algae, which indicated that studies of abiotic factors inducing plasticity were much more common (Padilla and Savedo, 2013). The prevalence of predator-induced plasticity in our review is perhaps not unexpected, given the well-established role of the gastropod shell in the evolution of anti-predator defense. However, it should be noted that several studies have shown plastic responses in gastropod traits to environmental factors other than predators. For example, soft tissue growth and shell shape in response to resource availability (for example, Rollo and Hawryluk, 1988; Bourdeau, 2010), conspecific density (for example, Kemp and Bertness, 1984), snail growth in response to the presence of heterospecific competitors (for example, Kawata and Ishigami, 1992), foot size and shell shape in response to hydrodynamic forces (for example, Etter, 1988; Trussell, 1997; Hollander et al., 2006), and life history traits in response to temperature (for example, Dybdahl and Kane, 2005; Melatunan et al., 2013). Somewhat surprisingly, our review turned up no examples of studies of shell or foot size plasticity in response to hydrodynamic forces in freshwater systems. This is surprising as the studies on freshwater taxa were carried out on species that are also common in rivers. However, there is some evidence to suggest that such plastic responses may occur in species that inhabit both moving and standing-water habitats (see Dillon et al., 2013 for an example of ‘cryptic plasticity’ in gastropod shells due to current in rivers). Further, populations of Lymnaea peregra from moving water have been observed to have larger aperture length to shell length ratios than populations from standing water and this shell shape feature was found to be genetically determined for only some populations, suggesting plasticity in others (Lam and Calow, 1988).
More studies examining the effect of abiotic factors on shell plasticity in aquatic gastropods are needed. Hydrodynamic factors may be particularly important, especially within the context of predator-induced shell defenses, because the shell responses necessary to defend against predators (that is, increased globosity, a thicker shell and narrow aperture) are often traded off against those needed to perform well under hydrodynamic stress (that is, a thinner, more elongate shell with wider aperture to accommodate a larger foot). Such a tradeoff has been observed several times in marine systems (for example, Etter, 1988; Palmer, 1990; Trussell, 2000a, b), but may also be important in some freshwater environments, particularly river or stream habitats or the shorelines of large lakes. For example, the costs of moving upstream in flowing water will be greater in spinose, large or thick-shelled morphs because of increased drag. Moreover, any advantage of reduced vulnerability to predators may be traded off against increased vulnerability to turbulent storm flows or wave surges (for example, Holomuzki and Biggs, 2006). Studies focused on the adaptive trade-offs of plastic responses to conflicting selective pressures (such as predators and hydrodynamic stress) may uncover hidden costs associated with the expression of specific phenotypes (for example, DeWitt et al., 2000; Langerhans and DeWitt, 2002; Bourdeau, 2009) and also indicate limitations to the adaptive value of the range of phenotypes that a species can express (for example, Langerhans and DeWitt, 2002; Hoverman and Relyea, 2007).
Summary
Our meta-analysis indicated large differences in the magnitude of plastic responses between marine and freshwater gastropods. We hypothesise that these differences may reflect limited calcium availability in freshwater habitats and the potential for higher associated metabolic expenses of accumulating, transporting and precipitating CaCO3, and producing the organic shell matrix. However, more work quantifying environmental constraints and energetic costs on shell production will be necessary for elucidating the mechanisms underlying our findings. We did not find differences in magnitude of plastic responses between marine taxa with planktonic or benthic development, suggesting that larval dispersal may not predict higher magnitude of phenotypic plasticity. Given the documented role of phenotypic plasticity in explaining patterns of intraspecific variation in aquatic gastropods (for example, Kemp and Bertness, 1984; Urabe, 1998; Trussell and Smith, 2000) and the cascading effects of phenotypic plasticity expressed by aquatic gastropods on species interactions and community structure (Turner et al., 2000; Trussell et al., 2002; Trussell et al., 2003), we envision a profitable future for the study of phenotypic plasticity in this system, which can be a major contributor to the wider understanding of the evolution of plasticity.
Data Archiving
Data used in the meta-analysis are archived in the Dryad repository: http://dx.doi.org/10.5061/dryad.ck2d2.
References
Adams DC, Gurevitch J, Rosenberg MS . (1997). Resampling tests for meta-analysis of ecological data. Ecology 78: 1277–1283.
Agrawal AA . (2001). Phenotypic plasticity in the interactions and evolution of species. Science 294: 321–326.
Appleton RD, Palmer AR . (1988). Water-borne stimuli released by predatory crabs and damaged prey induce more predator-resistant shells in a marine gastropod. Proc Natl Acad Sci USA 85: 4387–4391.
Auld JR, Agrawal AA, Relyea RA . (2009). Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc R Soc B 277: 503–511. rspb20091355.
Auld JR, Relyea RA . (2011). Adaptive plasticity in predator-induced defenses in a common freshwater snail: altered selection and mode of predation due to prey phenotype. Evol Ecol 25: 189–202.
Behrens Yamada S . (1989). Are direct developers more locally adapted than planktonic developers? Mar. Biol. 103: 403–411.
Berrigan D, Scheiner SM . (2004) Modeling the evolution of phenotypic plasticity. In: DeWitt TJ, Scheiner TJ, SM (eds). Phenotypic Plasticity: Functional and Conceptual Approaches. Oxford, Univ. Press: New York, pp 82–97.
Bertness MD, Garrity SD, Levings SC . (1981). Predation pressure and gastropod foraging: a tropical-temperate comparison. Evolution 35: 995–1007.
Bibby R, Cleall-Harding P, Rundle SD, Widdicombe S, Spicer J . (2007). Ocean acidification disrupts induced defences in the intertidal gastropod Littorina littorea. Biol Lett 3: 699–701.
Bourdeau PE . (2009). Prioritized phenotypic responses to combined predators in a marine snail. Ecology 90: 1659–1669.
Bourdeau PE . (2010). An inducible morphological defence is a passive by-product of behaviour in a marine snail. Proc R Soc B 277: 455–462.
Bourdeau PE . (2011). Constitutive and inducible defensive traits in co-occurring marine snails distributed across a vertical rocky intertidal gradient. Funct Ecol 25: 177–185.
Bourdeau PE . (2012). Intraspecific trait cospecialization of constitutive and inducible morphological defences in a marine snail from habitats with different predation risk. J Anim Ecol 81: 849–858.
Brönmark C, Lakowitz T, Hollander J . (2011). Predator-induced morphological plasticity across local populations of a freshwater snail. PLoS One 6: e21773.
Brookes JI, Rochette R . (2007). Mechanism of a plastic phenotypic response: predator-induced shell thickening in the intertidal gastropod Littorina obtusata. J Evol Biol 20: 1015–1027.
Brown JS . (1990). Habitat selection as an evolutionary game. Evolution 44: 732–746.
Bukowski SJ, Auld JR . (2014). The effects of calcium in mediating the inducible morphological defences of a freshwater snail. Physa Acuta Aquat Ecol 48: 85–90.
Chase JM . (1999). To grow or to reproduce? the role of life-history plasticity in food web dynamics. Am Nat 154: 571–586.
Clarke AH . (1978) Polymorphism in Marine Mollusks and Biome Development. Smithsonian Institution Press: Washington, DC, USA.
Cotton PA, Rundle SD, Smith KE . (2004). Trait compensation in marine gastropods: shell shape, avoidance behavior, and susceptibility to predation. Ecology 85: 1581–1584.
Crowl TA, Covich AP . (1990). Predator-induced life-history shifts in a freshwater snail. Science 247: 949–951.
Delgado GA, Glazer RA, Stewart NJ . (2002). Predator-Induced behavioral and morphological plasticity in the tropical marine gastropod Strombus gigas. Biol Bull 203: 112–120.
Denny MW, Paine RT . (1998). Celestial mechanics, sea-level changes, and intertidal ecology. Biol Bull 194: 108–115.
DeWitt TJ . (1998). Costs and limits of phenotypic plasticity: Tests with predator-induced morphology and life history in a freshwater snail. J Evol Biol 11: 465–480.
DeWitt TJ, Sih A, Wilson DS . (1998). Costs and limits of phenotypic plasticity. Trends Ecol Evol 13: 77–81.
DeWitt TJ, Sih A, Hucko JA . (1999). Trait compensation and cospecialization in a freshwater snail: size, shape and antipredator behaviour. Anim Behav 58: 387–407.
DeWitt TJ, Robinson BW, Wilson DS . (2000). Functional diversity among predators of a freshwater snail imposes an adaptive trade-off for shell morphology. Evol Ecol Res 2: 129–148.
DeWitt TJ, Scheiner SM (eds). (2004) Phenotypic Plasticity: Functional and Conceptual Approaches. Oxford University Press: Oxford: UK, pp 98–111.
Dillon R, Jacquemin SJ, Pyron M . (2013). Cryptic phenotypic plasticity in populations of the freshwater prosobranch snail, Pleurocera canaliculata. Hydrobiologia 709: 117–127.
Donald KM, Keeney DB, Spencer HG . (2011). Contrasting population makeup of two intertidal gastropod species that differ in dispersal opportunities. J Exp Mar Biol Ecol 396: 224–232.
Dybdahl MF, Kane SL . (2005). Adaptation vs phenotypic plasticity in the success of a clonal invader. Ecology 86: 1592–1601.
Edgell TC, Neufeld CJ . (2008). Experimental evidence for latent developmental plasticity: Intertidal whelks respond to a native but not an introduced predator. Biol Lett 4: 385–387.
Edgell TC, Rochette R . (2009). Prey-induced changes to a predator's behaviour and morphology: Implications for shell–claw covariance in the northwest Atlantic. J Exp Mar Biol Ecol 382: 1–7.
Edgell TC, Lynch BR, Trussell GC, Palmer AR . (2009). Experimental evidence for the rapid evolution of behavioral canalization in natural populations. Am Nat 174: 434–440.
Edgell TC, Hollander J . (2011) The evolutionary ecology of European Green Crab, Carcinus maenas, in North America. In: Galil BS, Clark PF, Carlton JT (eds). In the Wrong Place – Alien Marine Crustaceans: Distribution, Biology and Impacts. Springer: Berlin: Berlin, pp 641–659.
Etter RJ . (1988). Asymmetrical developmental plasticity in an intertidal snail. Evolution 42: 322–334.
Figuerola J, Green AJ . (2002). Dispersal of aquatic organisms by waterbirds: a review of past research and priorities for future studies. Freshw Biol 47: 483–494.
Ghalambor CK, McKay JK, Carroll SP, Reznick DN . (2007). Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21: 394–407.
Gurevitch J, Morrow LL, Wallace A, Walsh JS . (1992). A meta-analysis of competition in field experiments. Am Nat 140: 539–572.
Gurevitch J, Hedges LV . (1993) Meta-analysis: combining the results of independent experiments. In: Scheiner SM, Gurevitch J (eds). Design and Analysis of Ecological Experiments. Chapman and Hall: New York, pp 378–398.
Highsmith RC . (1985). Floating and algal rafting as potential dispersal mechanisms in brooding invertebrates. Mar Ecol Progr Ser 25: 169–179.
Hollander J, Butlin RK . (2010). The adaptive value of phenotypic plasticity in two ecotypes of a marine gastropod. BMC Evol Biol 10: 333.
Hollander J, Collyer ML, Adams DC, Johannesson K . (2006). Phenotypic plasticity in two marine snails: constraints superseding life history. J Evol Biol 19: 1861–1872.
Hollander J . (2008). Testing the grain-size model for the evolution of phenotypic plasticity. Evolution 62: 1381–1381.
Holomuzki JR, Biggs BJF . (2006). Habitat-specific variation and performance trade-offs in shell armature of New Zealand mudsnails. Ecology 87: 1038–1047.
Hoverman JT, Auld JR, Relyea RA . (2005). Putting prey back together again: integrating predator-induced behavior, morphology, and life history. Oecologia 144: 481–491.
Hoverman JT, Relyea RA . (2007). How flexible is phenotypic plasticity? developmental windows for trait induction and reversal. Ecology 88: 693–705.
Hoverman JT, Relyea RA . (2009). Survival trade-offs associated with inducible defences in snails: the roles of multiple predators and developmental plasticity. Funct Ecol 23: 1179–1188.
Hoverman JT, Cothran RD, Relyea RA . (2014). Generalist versus specialist strategies of plasticity: snail responses to predators with different foraging modes. Freshw Biol 59: 1101–1112.
Kawata M, Ishigami H . (1992). The growth of juvenile snails in water conditioned by snails of a different species. Oecologia 91: 245–248.
Kemp P, Bertness MD . (1984). Snail shape and growth rates – evidence for plastic shell allometry in Littorina littorea. Proc Nat Acad Sci USA 81: 811–813.
Kishida O, Mizuta Y, Nishimura K . (2006). Reciprocal phenotypic plasticity in a predator-prey interaction between larval amphibians. Ecology 87: 1599–1604.
Kopp M, Tollrian R . (2003). Reciprocal phenotypic plasticity in a predator–prey system: inducible offences against inducible defences? Ecol Lett 6: 742–748.
Lam PKS, Calow P . (1988). Differences in the shell shape of Lymnaea peregra (Muller) (Gastropoda, Pulmonata) from lotic and lentic habitats – environmental or genetic variance. J Mollusc Stud 54: 197–207.
Langerhans RB, DeWitt TJ . (2002). Plasticity constrained: over-generalized induction cues cause maladaptive phenotypes. Evol Ecol Res 4: 857–870.
Levins R . (1968) Evolution in Changing Environments. Princeton University Press: Princeton, NJ, USA.
Martel A, Chia FS . (1991). Drifting and dispersal of small bivalves and gastropods with direct development. J Exp Mar Biol Ecol 150: 131–147.
Martín-Mora E, James FC, Stoner AW . (1995). Developmental plasticity in the shell of the queen conch Strombus gigas. Ecology 76: 981–994.
Melatunan S, Calosi P, Rundle SD, Widdicombe S, Moody AJ . (2013). Effects of ocean acidification and elevated temperature on shell plasticity and its energetic basis in an intertidal gastropod. Mar Ecol Progr Ser 472: 155–168.
Minton RL, Reese SA, Swanger K, Perez KE, Hayes DM . (2007). Changes in shell morphology of Elimia comalensis (Gastropoda: Pleuroceridae) from the Edwards Plateau, Texas. Southwest Nat 52: 475–481.
Minton RL, Lewis EM, Netherland B, Hayes DM . (2011). Large differences over small distances: plasticity in the shells of Elimia potosiensis (Gastropoda: Pleuroceridae). Int J Biol 3: 23–32.
Padilla DK, Savedo MM . (2013). A systematic review of phenotypic plasticity in marine invertebrate and plant systems. Adv Mar Biol 65: 67–94.
Palmer AR . (1983). Relative cost of producing skeletal organic matrix versus calcification: evidence from marine gastropods. Mar Biol 75: 287–292.
Palmer AR . (1990). Effect of crab effluent and scent of damaged conspecifics on feeding, growth, and shell morphology of the Atlantic dogwhelk Nucella lapillus (L.). Hydrobiologia 193: 155–182.
Palmer AR . (1992). Calcification in marine molluscs: How costly is it? Proc Natl Acad Sci USA 89: 1379–1382.
Palmer AR . (1999). Detecting publication bias in meta-analyses: A case study of fluctuating asymmetry and sexual selection. Am Nat 154: 220–233.
Parsons KE . (1997). Role of dispersal ability in the phenotypic differentiation and plasticity of two marine gastropods. Oecologia 110: 461–471.
Peacor SD, Werner EE . (1997). Trait-mediated indirect interactions in a simple aquatic food web. Ecology 78: 1146–1156.
Pernet B . (2007). Determinate growth and variable size at maturity in the marine gastropod Amphissa columbiana. Am Malacol Bull 22: 7–15.
Pfennig DW, Wund MA, Snell-Rood EC, Cruickshank T, Schlichting CD, Moczek AP . (2010). Phenotypic plasticity’s impacts on diversification and speciation. Trends Ecol Evol 25: 459–467.
Pigliucci M . (2003). Phenotypic integration: studying the ecology an evolution of complex phenotypes. Ecol Lett 6: 265–272.
Pigliucci M, Murren CJ, Schlichting CD . (2006). Phenotypic plasticity and evolution by genetic assimilation. J Exp Biol 209: 2362–2367.
Poitrineau K, Brown SP, Hochberg ME . (2004). The joint evolution of defence and inducibility against natural enemies. J Theor Biol 231: 389–396.
Price TD, Qvarnström A, Irwin DE . (2003). The role of phenotypic plasticity in driving genetic evolution. Proc R Soc B 270: 1433–1440.
Raup DM . (1961). The geometry of coiling in gastropods. Proc Nat Acad Sci USA 47: 602–609.
Richards CL, Bossdorf O, Muth NZ, Gurevitch J, Pigliucci M . (2006). Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol Lett 9: 981–993.
Rollo CD, Hawryluk MD . (1988). Compensatory scope and resource allocation in two species of aquatic snails. Evolution 69: 146–156.
Rosenberg MS, Adams DC, Gurevitch J . (1997) MetaWin. Statistical Software for Meta-analysis with Resampling Tests. Sinauer: Sunderland, MA, USA.
Rosenthal R . (1979). The ‘file drawer problem’ and tolerance for null results. Psychol Bull 86: 838–641.
Rosenthal R . (1984) Meta-analytic Procedures for Social Research. Sage: Beverly Hills, CA, USA.
Rosenthal R, Rosnow RL, Rubin DB . (2000) Contrasts and Effect Sizes in Behavioral Research: A Correlational Approach. Cambridge University Press: Cambridge, UK.
Rundle SD, Brönmark C . (2001). Inter–and intraspecific trait compensation of defence mechanisms in freshwater snails. Proc R Soc B 268: 1463–1468.
Rundle SD, Spicer JJ, Coleman RA, Vosper J, Soane J . (2004). Environmental calcium modifies induced defenses in snails. Proc Royal Soc B 271: S67–S70.
Sobel AH . (2012). Tropical Weather. Nature Education Knowledge 3: 2.
Tollrian R, Harvell CD . (1999) The Ecology and Evolution of Inducible Defenses. Princeton University Press: Princeton, NJ, USA.
Trussell GC . (1996). Phenotypic plasticity in an intertidal snail: the role of a common crab predator. Evolution 50: 448–454.
Trussell GC . (1997). Phenotypic plasticity in the foot size of an intertidal snail. Ecology 78: 1033–1048.
Trussell GC . (2000a). Phenotypic clines, plasticity, and morphological trade-offs in an intertidal snail. Evolution 54: 151–166.
Trussell GC . (2000b). Predator-induced morphological trade-offs in latitudinally-separated populations of Littorina obtusata. Evol Ecol Res 2: 803–822.
Trussell GC, Smith LD . (2000). Induced defenses in response to an invading crab predator: an explanation of historical and geographic phenotypic change. Proc Natl Acad Sci USA 97: 2123–2127.
Trussell GC, Ewanchuk PJ, Bertness MD . (2002). Field evidence of trait-mediated indirect interactions in a rocky intertidal food web. Ecol Lett 5: 241–245.
Trussell GC, Ewanchuk PJ, Bertness MD . (2003). Trait-mediated interactions in rocky intertidal food chains: predator risk cues alter prey feeding rates. Ecology 84: 629–640.
Turner AM, Mittelbach GG . (1990). Predator avoidance and community structure: interactions among piscivores, planktivores, and plankton. Ecology 71: 2241–2254.
Turner AM, Bernot RJ, Boes CM . (2000). Chemical cues modify species interactions: the ecological consequences of predator avoidance by freshwater snails. Oikos 88: 148–158.
Urabe M . (1998). Contribution of genetic and environmental factors to shell shape variation in the lotic snail Semisulcospira reiniana (Prosobranchia: Pleuroceridae). J Moll Stud 64: 329–343.
Van Leeuwen CHA, Huig N, Van der Velde G, Van Alen TA, Wagemaker CAM, Sherman CDH et al. (2013). How did this snail get here? Several dispersal vectors inferred for an aquatic invasive species. Freshw Biol 58: 88–99.
Van Leeuwen CHA . (2012). Experimental quantification of long distance dispersal potential of aquatic snails in the gut of migratory birds. Plos One 7: e32292.
Via S, Lande R . (1985). Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 39: 505–522.
Van Buskirk J . (2002). A comparative test of the adaptive plasticity hypothesis: relationships between habitat and phenotype in anuran larvae. Am Nat 160: 87–102.
Vermeij GJ . (1974). Marine faunal dominance and molluscan shell form. Evolution 28: 656–664.
Vermeij GJ . (1978) Biogeography and Adaptaton. Patterns of Marine Life. Harvard University Press: Cambridge, MA, USA, pp 332.
Vermeij GJ . (1982a). Environmental change and the evolutionary history of the periwinkle Littorina littorea in North America. Evolution 36: 561–580.
Vermeij GJ . (1982b). Phenotypic evolution in a poorly dispersing snail after arrival of a predator. Nature 299: 349–350.
Vermeij GJ . (1987) Evolution and Escalation: An Ecological History of Life. Princeton University Press: Princeton, NJ, USA, p 527.
Vermeij GJ . (2005) Shells Inside Out: the Architecture, Evolution and Function of Shell Dnvelopment in Molluscs. Evolving Form and Function: Fossils and Development. Peabody Museum of Natural History, Yale University: New Haven, CT, pp 197–221.
Wesselingh FP, Cadée GC, Renema W . (1999). Flying high: on the airborne dispersal of aquatic organisms as illustrated by the distribution histories of the gastropod genera Tryonia and Planorbarius. Geol Mijnbouw 78: 165–174.
West-Eberhard MJ . (1989). Phenotypic plasticity and the origins of diversity. Ann Rev Ecol Syst 20: 249–278.
West-Eberhard MJ . (2005). Developmental plasticity and the origin of species differences. Proc Nat Acad Sci USA 102: 6543–6549.
Yeh PJ, Price TD . (2004). Adaptive phenotypic plasticity and the successful colonization of a novel environment. Am Nat 164: 531–542.
Acknowledgements
We thank T DeWitt, G Vermeij and two anonymous reviewers for constructive criticism on earlier drafts of this manuscript. JH was funded by a Marie Curie European Reintegration Grant (PERG08-GA-2010-276915) and the Royal Physiographic Society in Lund. CB and RKB were supported by the Swedish Research Council and NERC, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Additional information
Supplementary Information accompanies this paper on Heredity website
Supplementary information
Rights and permissions
About this article
Cite this article
Bourdeau, P., Butlin, R., Brönmark, C. et al. What can aquatic gastropods tell us about phenotypic plasticity? A review and meta-analysis. Heredity 115, 312–321 (2015). https://doi.org/10.1038/hdy.2015.58
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hdy.2015.58
This article is cited by
-
Geometric morphometrics reveal complex shape variation patterns at different geographic scales in the patagonian gastropod Trophon geversianus
Evolutionary Ecology (2021)
-
Adaptive divergence in shell morphology in an ongoing gastropod radiation from Lake Malawi
BMC Evolutionary Biology (2020)
-
Phylogenetics and species delimitations of the operculated land snail Cyclophorus volvulus (Gastropoda: Cyclophoridae) reveal cryptic diversity and new species in Thailand
Scientific Reports (2019)
-
Cue specificity of predator-induced phenotype in a marine snail: is a crab just a crab?
Marine Biology (2019)
-
Species-specific responses to ocean acidification should account for local adaptation and adaptive plasticity
Nature Ecology & Evolution (2017)