Abstract
The life history strategies of males and females are often divergent, creating the potential for sex differences in selection. Deleterious mutations may be subject to stronger selection in males, owing to sexual selection, which can improve the mean fitness of females and reduce mutation load in sexual populations. However, sex differences in selection might also maintain sexually antagonistic genetic variation, creating a sexual conflict load. The overall impact of separate sexes on fitness is unclear, but the net effect is likely to be positive when there is a large sex difference in selection against deleterious mutations. Parasites can also have sex-specific effects on fitness, and there is evidence that parasites can intensify the fitness consequences of deleterious mutations. Using lines that accumulated mutations for over 60 generations, we studied the effect of the pathogenic bacterium Pseudomonas aeruginosa on sex differences in selection in the fruit fly Drosophila melanogaster. Pseudomonas infection increased the sex difference in selection, but may also have weakened the intersexual correlation for fitness. Our results suggest that parasites may increase the benefits of sexual selection.
Similar content being viewed by others
Introduction
The maintenance of sex and outcrossing are long-standing problems in evolutionary biology because these reproductive modes are widespread despite the inherent costs (Otto, 2009). Multiple factors have been proposed that may reduce the costs of sex, including the purging of deleterious mutations (Kondrashov, 1988) and coevolution with parasites (Hamilton et al., 1990; Agrawal, 2006). However, the explanatory power of such models is often limited to specific genetic or ecological scenarios (West et al., 1999; Otto, 2009). For example, sex may allow deleterious alleles to be purged more efficiently, but this requires synergistic epistasis among deleterious alleles, a pattern for which there is little evidence (de Visser and Elena, 2007). In addition, although antagonistic coevolution with parasites and other forms of ecological fluctuation can select for the genetic diversity produced by sex, it could also select for diversity among asexual clones (Howard and Lively, 1994). In general, theoretical models find that sex is favored by coevolution with parasites only under limited circumstances, suggesting that parasites may represent, at best, a partial explanation for sex (Howard and Lively, 1994; Agrawal, 2006).
The limited applicability of individual models has driven a shift in focus towards ‘pluralistic’ theories, which propose that multiple genetic and ecological factors working in concert may expand the conditions under which sexual populations can resist invasion by asexual clones (West et al., 1999; de Visser and Elena, 2007). Some of the restrictive assumptions of the mutational and ecological models can be relaxed when interactions between different factors are considered. For example, theory suggests that sex can be maintained when parasites and mutations have synergistic effects on fitness, even in the absence of synergistic epistasis among mutations (Park et al., 2010). Parasites have been found to increase the deleterious effects of mutations in bacteria (Cooper et al., 2005; Buckling et al., 2006), female Daphnia (Killick et al., 2006) and Drosophila larvae (Young et al., 2009; Figure 1a).
Conceptual basis for studying the effect of parasites on sex-specific selection. There is empirical evidence for synergistic effects of parasites and mutations (A), sex-specific effects of mutations (B), and sex differences in immunity and the effect of parasites on fitness (C), leading to the prediction that parasites and mutations may interact in a sex-specific manner, i.e. a three-way interaction.
Another idea for the maintenance of sex that has recently received increased attention concerns the pattern of selection acting against deleterious mutations in males and females. Stronger selection on males resulting from sexual selection is expected to lessen the impact of deleterious mutations on the mean fitness of females, increase the fitness of sexually produced offspring relative to asexually produced offspring, and thus favor sex (Whitlock and Agrawal, 2009; Roze and Otto, 2011). Studies of phenotypic marker mutations (Whitlock and Bourguet, 2000; Pischedda and Chippindale, 2005; Sharp and Agrawal, 2008) and spontaneous mutations (Mallet et al., 2011; Sharp and Agrawal, 2012a) suggest that sexual selection has the potential to reduce female mutation load (Figure 1b). For example, Mallet et al., (2011) and Sharp and Agrawal (2012a) studied the fitness of Drosophila melanogaster chromosomes subjected to MA when expressed in males and females. Despite the differences in methodology (for example, the use of the X chromosome versus an autosome), in both cases mutations were ~1.5 times more deleterious in males than in females. Importantly, both studies also identified a positive intersexual genetic correlation among lines, indicating that mutations generally affected fitness in both sexes, that is, their effects were sexually concordant (Connallon et al., 2010). Stronger selection on males is therefore expected to purge mutations that are deleterious to females, and reduce the mutation load of sexual females relative to asexuals.
However, experimental evolution studies that manipulate the strength of sexual selection find mixed evidence for a net benefit to female fitness (Whitlock and Agrawal, 2009; Hollis and Houle, 2011; McGuigan et al., 2011; Arbuthnott and Rundle, 2012). One possible explanation is that, although sex-specific selection may reduce mutation load, it is also likely to generate conflict between the sexes. The net effect of sex-specific selection will therefore depend on both the extent of sexual conflict and the strength of purifying selection in each sex (Connallon et al., 2010). In the presence of sexually antagonistic selection, the maintenance of sexual reproduction is more likely when the difference in selection on deleterious mutations between males and females is larger (Roze and Otto, 2011). In the spirit of pluralistic approaches, we asked what ecological factors might increase the sex difference in selection, increasing the likelihood that sex will persist.
We hypothesized that the magnitude of sex-specific selection could be affected by the presence of parasites. Males and females often differ in their immune investment and the degree to which their fitness is affected by parasites (for example, McKean and Nunney, 2005; Winterhalter and Fedorka, 2008; Imroze and Prasad, 2011; Duneau et al., 2012; Nystrand and Dowling, 2014; Vincent and Sharp, 2014; Figure 1c). It is therefore plausible that the synergistic effects of parasites and mutations could be sex specific. In other words, given that parasites, mutations and sex differences each interact in a pairwise fashion (Figure 1), we might expect an interaction between the three factors. This might be particularly true if immune function is an honest signal of genetic quality (that is, the presence or absence of deleterious mutations) and is targeted by sexual selection (Hamilton and Zuk, 1982; Blount et al., 2003; López and Martín, 2005).
We explored this idea by examining the effect of parasites on sex differences in selection. We measured the effects of mutations on D. melanogaster of each sex when inoculated with the bacterial pathogen Pseudomonas aeruginosa or with sterile media. Our results suggest that parasites can increase the difference in selection between the sexes, which could increase the explanatory power of the sexual selection hypothesis for the maintenance of sex.
Materials and methods
Fly husbandry and experimental lines
All flies were reared in shell vials at 25 °C, 70% relative humidity, with a 12L:12D cycle, on yeast-sugar-agar media. Flies were handled under CO2 anesthesia, and virgin flies used for experiments were housed in separate-sex vials at a density of 25 flies per vial.
We studied lines that were subjected to mutation accumulation (MA) and nonmutant controls. In an MA experiment, replicate lines that are initially genetically identical are bottlenecked independently for many generations, preventing selection against most new mutations, allowing them to fix in the genome. These lines can be compared with controls of the same genotype that have not been bottlenecked, where selection should limit MA. The experimental lines used in this study were a subset of lines described elsewhere (Sharp and Agrawal, 2012a, 2012b). All MA lines shared an initially identical copy of chromosome 2 (~40% of the genome) and accumulated mutations in the heterozygous state. Three control populations of 450 adults each were also generated at the beginning of the experiment using the same progenitor chromosome as the MA lines.
For this study we considered only homozygous-viable MA lines that accumulated mutations on a wild-type genetic background. Following 62 generations (~29 months) of MA we obtained flies for fitness assays from 25 MA lines by conducting 5 generations of crosses to situate homozygous MA chromosomes on an isogenic wild-type background, using standard marker and balancer stocks to track chromosomes and suppress recombination. Similar crosses were performed for 25 chromosomes sampled from the control populations, where each chromosome was bottlenecked and situated on the same isogenic background. These crosses served to eliminate all genetic variation within the lines, as well as the variation among lines that was not due to mutations that accumulated in MA lines or were segregating in control populations, and any variation on the tiny fourth chromosome, which was not manipulated.
Ideally, fitness in the control populations would represent that of the un-mutated common ancestor of the MA lines. However, although selection should have limited the spread of new mutations in the control populations, it is likely that some new deleterious alleles were present among the control chromosomes we sampled. If most mutations have recessive effects, then heterozygous control chromosomes may better represent ancestral fitness (as in Sharp and Agrawal, 2012a). Nevertheless, we chose to compare homozygous MA lines with homozygous controls, because heterozygosity is known to influence invertebrate immunity (Rantala and Roff, 2007). Thus, control lines may have been carrying homozygous deleterious alleles on chromosome 2 at a frequency that reflects (the approach to) mutation–selection balance, whereas the MA lines should have become enriched for homozygous deleterious alleles on chromosome 2. Otherwise, all lines were effectively genetically identical.
Inoculations
One day before inoculations, a single colony of P. aeruginosa (strain PA01) was grown overnight in LB broth at 37 °C. The overnight culture was diluted so that the optical density at 600 nm (OD600 nm) was <0.05 and allowed to grow for ~5 h, corresponding to the log phase of growth (C Vincent, unpublished data). The culture (1ml) was centrifuged and resuspended in MgSO4 solution. Absorbance at OD600 nm was then measured on a spectrophotometer and the desired final concentration was obtained through serial dilution. Two doses with detectable effects on fly survivorship were selected based on preliminary assays, corresponding to OD600 nm of 0.001 and 0.002, henceforth D1 and D2, respectively. Flies were infected following the injector pumping method (Apidianakis and Rahme, 2009). One day before the fitness assay each focal fly was injected with one of the three inoculants: a ‘sham’ of sterile MgSO4 solution or one of the two doses of P. aeruginosa.
A subset of infected and sham flies were homogenized and plated immediately following inoculation; these plates were incubated for ~16 h at 37 °C and examined for the presence of bacterial colonies. On average, 1.85 and 2.75 log10[x+1] colonies were observed for D1 (N=28, s.e.=0.13) and D2 (N=25, s.e.=0.09), respectively, representing relatively low doses that should mimic the early stages of infection (Apidianakis and Rahme, 2009). Bacteria were absent on all plates from the uninfected (sham) treatment, indicating that our inoculation method was free from contamination. These quantitative estimates of each dose were used in subsequent data analyses.
Virgin flies (5-6 days post eclosion) were injected midway along the dorsolateral line of the thorax with 9.6 nl of inoculum using a Nanoject microprocessor-controlled microinjection pipette Drummond Scientific (Broomall, PA, USA) with a pulled-glass capillary tip. This method results in a systemic infection with a precise dose of bacteria, although minimizing wounding relative to other methods (Apidianakis and Rahme, 2009). Inoculations were performed in random order with respect to mutation treatment, alternating frequently between the sexes. After one day, surviving flies were placed in mating groups for fitness assays as described below. Measures of bacterial load (Vincent and Sharp, 2014) indicate that bacteria were still present in all flies inoculated with D1 or D2 at this time, that is, flies did not clear the infection before the fitness assays.
Fitness assays
To assess adult fitness, inoculated ‘focal’ flies were placed in mating groups with one fly of the same sex and two flies of the opposite sex (see Supplementary Figure S1). The ‘focal’ MA and control chromosomes carry the recessive marker bw, designated bw*. To assess male fitness, in each mating group one focal male (bw*/bw*) and one wild-type male (+/+) were housed with two outbred bw/bw females. To assess female fitness, in each mating group one focal female (bw*/bw*) and one wild-type female (+/+) were housed with two outbred bw/bw males. All flies were virgins. The offspring of focal individuals will be bw*/bw and express a brown eye color phenotype as adults, whereas the offspring produced by non-focal wild-type flies will be +/bw and express a red eye color phenotype. We considered the number of offspring produced by both focal and non-focal individuals, and defined the absolute fitness of a given focal individual as the number of focal (brown eyed) offspring eclosed, nfocal, relative to the total number of offspring eclosed, ntotal=nfocal+nnon-focal. The total number of offspring in each vial was incorporated to reduce variation in fitness measures owing to variation in food quality among vials and to account for variation in the productivity of the standard bw/bw flies. Multiple replicate mating groups (>5 on average) were initiated for each combination of sex, genotype and infection level (sham, D1, D2). Flies were given 3 days to interact and produce offspring. Offspring were scored for eye color on days 12 and 15 following vial initiation and these scores were summed. Over 175 000 offspring were scored from 1749 replicate mating trials.
Data analysis
Under MA, mean fitness declines at a rate ΔM=UE[s], where U is the deleterious mutation rate and E[s] is average selection against new mutations. ΔM can be estimated from the fitness (W) of MA and control lines as (1−WMA/Wcontrol)/t, where t is the number of generations of MA. When males and females share mutations, ψ=ΔMmale−ΔMfemale reflects the sum of sex differences in selection across mutations. Our goal was to test for differences in the sex difference in selection, ψ, across infection levels. We first modeled mean fitness in each experimental group (the crossed factors of MA/control, sex and infection level), using maximum likelihood implemented in R (R Core Team, 2013). We considered the number of focal offspring, nfocal, and the total number of offspring, ntotal=nfocal+nnon-focal. In a given group, we modeled the distribution of line means as a beta distribution with mean μ and variance σ and replicates within lines as a beta-binomial distribution with mean x and dispersion ρ. For a group the log likelihood is given by

where the summation is over all lines, i, the integration is over all possible line means, x, and the product is over all replicates within a line, j. B represents the beta density function, and BB represents the beta-binomial density function for nfocal successes out of ntotal trials given probability of success x and dispersion ρ. The integration was simplified by using a discrete approximation to the beta distribution based on 51 equally weighted quantiles. Groups within each infection level were modeled such that the mean (μ) was given by μfemale for control females, μfemale(1−ΔMfemale) for mutant females, μmale for control males and μmale(1−ΔMmale)=μmale(1−(ΔMfemale+ψ)) for mutant males. We tested the null hypothesis of no effect of infection on the sex difference in selection by constraining ψ to be equal across infection levels. Nested models were compared using likelihood ratio tests (LRT) with one degree of freedom. We searched for optimal parameter values using the optim function; using random starting values we first applied the Nelder–Mead algorithm iteratively until improvements in log likelihood became small (<0.2) and then applied the Broyden–Fletcher–Goldfarb–Shanno algorithm. We conducted at least 10 and as many as 25 repeated optimizations using different random starting values to ensure the best parameters were obtained; most runs converged on similar values.
After testing for differences in average ψ among infection levels, we calculated ΔM and ψ for each MA line individually, at each infection level. For each line within a group we calculated W as Σnfocal/Σntotal, where the summations are over all replicates in the line, and ΔM as (1−W/μcontrol)/t, where μcontrol is the maximum likelihood value for control fitness in that group. We then used the lmer function in R to model the effects of sex and initial number bacteria (dose) on ΔM in a linear mixed model with a random effect of MA line on the intercept and slopes. Initial bacteria values were estimated as described in the methods. We used a similar approach to model the linear and quadratic effects of initial bacteria on ψ. Models were fit by maximum likelihood and compared using LRT with one degree of freedom. Pearson correlations of arcsine square root-transformed W values were used to assess the relationship between male and female fitness, and between infected and uninfected fitness.
Results
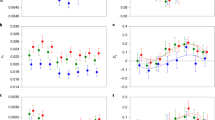
Maximum likelihood parameters are shown in Table 1. In the absence of infection, the sex difference in selection, ψ, was slightly negative and not significantly different from 0 (LRT; χ2=0.064, P=0.80). ψ increased at D1, but was not significantly different from the uninfected value (LRT; χ2=1.58, P=0.21). However, ψ became significantly larger and positive at D2 (LRT; χ2=5.95, P=0.015). In other words, the sex difference in selection became larger under infection, with stronger selection on males, but this effect was only significant at the higher infection level.
A mixed model analysis of fitness decline in individual MA lines revealed a similar pattern, with a significant sex-by-infection interaction effect on the rate of fitness decline, ΔM (LRT; χ2=4.34, P=0.037); this interaction is apparent in Figure 2. This pattern was also reflected by the sex difference in selection for individual MA lines, ψ. Relative to the uninfected group, mean ψ increased by 4.4 times in flies infected with D1 and by 18.1 times in flies infected with D2. We found that a quadratic effect of initial bacteria on ψ was a better fit to the data than a linear effect of initial bacteria based on comparisons of Akaike information criteria between the different mixed models. This quadratic term was positive and significant (LRT; χ2=4.54, P=0.033), and the same result was obtained using non-log-transformed values for initial bacteria. These results suggest that the sex difference in selection increased in an accelerating fashion with increasing initial dose of bacteria.
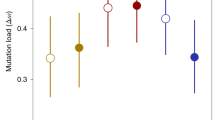
For sex differences in selection to reduce mutation load, mutations that are deleterious to females must be more strongly deleterious in males (Whitlock and Agrawal, 2009; Roze and Otto, 2011). If most mutations have a deleterious effect on both sexes, we expect a positive correlation in fitness between males and females across mutant lines. We observed a significant positive intersexual correlation for mutant fitness in the absence of infection (Figure 3; r=0.41, df=23, P=0.041). The intersexual correlations were positive but not significantly different from 0 in the presence of infection (Figure 3; D1: r=0.11, df=23, P=0.59; D2: r=0.08, df=23, P=0.71). However, there was no evidence that the intersexual correlation declined significantly between the infected and uninfected groups (uninfected vs D1: Z=1.06, P=0.29; uninfected vs D2, Z=1.19, P=0.24) or differed among all the three levels of infection (χ2=1.71, P=0.43).
Intersexual correlations for arcsine-transformed mutant W values in uninfected flies and flies infected at levels D1 and D2, with linear regression lines. We observed a significant positive intersexual correlation in uninfected flies but not in infected flies; the correlation coefficients do not differ significantly from one another.
Although we did not detect an intersexual correlation for fitness under infection, we found that fitness within each sex was positively correlated between the uninfected and infected groups, particularly for females (Supplementary Figure S2; all df=23; uninfected vs D1: females, r=0.53, P=0.007; males, r=0.32, P=0.116; uninfected vs D2: females, r=0.70, P<0.0001; males r=0.30, P=0.150), suggesting that the relative ranking of genotypes was consistent across environments. Finally, we tested for a correlation between infected male fitness and uninfected female fitness; this correlation was nonsignificant for D1 (r=–0.07, df=23, P=0.74), but significant and positive for D2 (r=0.46, df=23, P=0.021), suggesting that selection on infected males may reduce the mutation load of females in the absence of infection.
Discussion
In populations with separate sexes, there is reason to expect sex-specific effects of both new mutations and parasites owing to the divergent life history strategies of males and females. In D. melanogaster, there is evidence that mutations may generally have stronger effects on male fitness than on female fitness (Whitlock and Agrawal, 2009; Mallet et al., 2011; Sharp and Agrawal, 2012a). There are also examples in this species of sex differences in immune investment (McKean and Nunney, 2005; Winterhalter and Fedorka, 2008; Imroze and Prasad, 2011) and theoretical models predicting such differences (Stoehr and Kokko, 2006; Restif and Amos, 2010). In light of these previous findings it may be reasonable to expect mutations and parasites to interact in a sex-specific fashion in their effects on fitness. We tested this hypothesis by estimating the reproductive success of mutant and nonmutant males and females under different levels of infection. The reproductive success of focal individuals was measured relative to that of standard individuals from the same vial, which served as potential competitors for mates and food resources, and controlled for variation in resource quality among vials. We then compared these estimates between mutant and nonmutant lines to estimate the relative effect of mutations in each sex, for each infection level.
The lines studied here are known to harbor mutations with deleterious effects on larval viability (Sharp and Agrawal, 2012b), and stronger effects on adult male fitness than on adult female fitness (Sharp and Agrawal, 2012a). In this experiment the impact of mutations on fitness in uninfected flies was weaker than that observed previously; this might be due to environmental differences between the studies, but most likely reflects the fact that the control genotypes in this experiment were homozygous, and may have been expressing recessive deleterious alleles that were not present in the ancestor of the MA lines, leading us to underestimate the effects of new mutations. Nevertheless, our results provide some support for the idea that parasites could increase the sex difference in selection, which would reduce mutation load in populations with high parasite burdens. We observed a significant effect of P. aeruginosa infection on the sex difference in selection, particularly at the highest infection level. This could reflect a nonlinear interaction between sex and infection intensity; the results of another recent study suggest that such nonlinear sex-dependent effects of infection may be common (Nystrand and Dowling, 2014). However, our test for linearity included fitness estimates from the uninfected group, which assumes that the complete absence of infection can be treated as a point on the continuum of infection intensity.
Our approach does not allow us to identify the mechanism by which infection may have increased the sex difference in selection. If deleterious mutations compromise immunity and females choose mates on the basis of immunity, this could result in increased selection against infected mutant males. Although resistance to parasites may be an honest signal of good genes in some cases (Hamilton and Zuk, 1982), the evidence that females prefer immune competent males is mixed (Blount et al., 2003; López and Martín, 2005; Kortet et al., 2012). Infection could also have affected the strength of post-copulatory sexual selection if there is a genetic trade-off between immunity and sperm quality (Simmons and Roberts, 2005). However, post-copulatory sexual selection may be less important than pre-copulatory sexual selection in removing deleterious mutations (Clark et al., 2012). Finally, infection could have affected selection via other adult life history traits, such as survivorship, in a sex-specific manner. In any case, our data suggest that synergistic effects of parasites and mutations may occur in a sex-specific manner.
Although our fitness estimates potentially captured the effects of mutations on several life history components, it is likely that the main determinant of reproductive success was the competition for mates in males and fecundity in females. These measures are not necessarily equivalent to total selection, which is the most relevant metric in determining the benefit of sex-specific selection (Sharp and Agrawal, 2012a). For example, our experiment did not account for homozygous effects of mutations on larval viability. These effects could be exacerbated by parasites (Young et al., 2009), but larvae are likely subject to less sex-specific selection than adults in this species. Increased selection on viability or other non-sex-specific fitness components under infection would lead us to underestimate the net effect of mutations on fitness in the infected flies of both sexes. Our estimates of fitness decline may have been influenced by the heterozygous effects of mutations on viability, as we assessed fitness by scoring offspring that were heterozygous for the chromosome of interest. However, we expect this effect to be small, because mutations will generally have recessive effects. In the absence of maternal or paternal effects, the effect of selection on heterozygous offspring should be similar across sexes (because offspring were used to score male and female fitness in the same way) and similar across infection levels (because infection occurred in adults, but not their offspring).
The reduction in female mutation load owing to sex differences in selection is best estimated by comparing the total fitness decline in females to the average fitness decline across sexes. Using this method, previous studies indicate a per-locus reduction in load of ~20% (Mallet et al., 2011; Sharp and Agrawal, 2012a). Our data indicate a load reduction of ~13% in uninfected flies and ~78% in flies infected at D1. Unfortunately this metric is not meaningful for flies infected at D2, because our point estimate of fitness decline in females, ΔMfemale, is negative (Figure 2). This ostensibly indicates that mutations were beneficial in females and deleterious in males at infection level D2. Coupled with the lack of a significant intersexual correlation for fitness under infection, this result implies that infection may have caused some mutations to have sexually antagonistic effects on fitness.
Although we cannot rule out the possibility that some mutations had sexually antagonistic effects at the higher infection level, this explanation seems unlikely given the following considerations. First, as noted above, our estimates of control fitness may be downwardly biased, and mutations are likely to have deleterious effects on fitness components that were not measured in this study, leading us to underestimate the total deleterious effect of mutations. Thus, negative fitness decline does not necessarily reflect the presence of beneficial mutations. Second, although we failed to detect a significant positive intersexual correlation for fitness under infection, we found significant positive correlations across environments, both within and between sexes, suggesting that the rank ordering of genotypes was consistent between the infection levels. Finally, it is not clear why infection should reverse the sign of selection in females but not in males. We suspect that infection weakened selection on females, but did not increase the extent of sexual antagonism for fitness. However, our results are also consistent with the finding that the intersexual correlation for fitness may be difficult to predict in novel environments (Punzalan et al., 2013).
Most populations will be subject to the effects of both deleterious mutations and parasites. These ubiquitous forces are thought to favor the evolution of sex and outcrossing, both independently and in conjunction, and each is expected to act in a sex-specific fashion. We find evidence for sex-specific interactions between mutations and parasites, highlighting the value of pluralistic hypotheses for the maintenance of sexual reproduction. More studies are needed to explore the effects of parasites on both sexually concordant and sexually antagonistic selection.
Data archiving
Data have been deposited in Dryad, http://doi.org/10.5061/dryad.3jh4r.
References
Agrawal AF . (2006). Similarity selection and the evolution of sex: revisiting the red queen. Plos Biol 4: 1364–1371.
Apidianakis Y, Rahme LG . (2009). Drosophila melanogaster as a model host for studying Pseudomonas aeruginosa infection. Nat Protoc 4: 1285–1294.
Arbuthnott D, Rundle HD . (2012). Sexual selection is ineffectual or inhibits the purging of deleterious mutations in Drosophila melanogaster. Evolution 66: 2127–2137.
Blount JD, Metcalfe NB, Birkhead TR, Surai PF . (2003). Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science 300: 125–127.
Buckling A, Wei Y, Massey RC, Brockhurst MA, Hochberg ME . (2006). Antagonistic coevolution with parasites increases the cost of host deleterious mutations. Proc R Soc B 273: 45–49.
Clark SCA, Sharp NP, Rowe L, Agrawal AF . (2012). Relative effectiveness of mating success and sperm competition at eliminating deleterious mutations in Drosophila melanogaster. PLoS ONE 7: e37351.
Connallon T, Cox RM, Calsbeek R . (2010). Fitness consequences of sex-specific selection. Evolution 64: 1671–1682.
Cooper TF, Lenski RE, Elena SF . (2005). Parasites and mutational load: an experimental test of a pluralistic theory for the evolution of sex. Proc R Soc B 272: 311–317.
de Visser JAGM, Elena SF . (2007). The evolution of sex: empirical insights into the roles of epistasis and drift. Nat Rev Genet 8: 139–149.
Duneau D, Luijckx P, Ruder LF, Ebert D . (2012). Sex-specific effects of a parasite evolving in a female-biased host population. BMC Biology 10: 104.
Hamilton W, Zuk M . (1982). Heritable true fitness and bright birds: a role for parasites? Science 218: 384–387.
Hamilton W, Axelrod R, Tanese R . (1990). Sexual reproduction as an adaptation to resist parasites (a review). Proc Natl Acad Sci USA 87: 3566–3573.
Hollis B, Houle D . (2011). Populations with elevated mutation load do not benefit from the operation of sexual selection. J Evol Biol 24: 1918–1926.
Howard RS, Lively CM . (1994). Parasitism, mutation accumulation and the maintenance of sex. Nature 367: 554–557.
Imroze K, Prasad N . (2011). Sex-specific effect of bacterial infection on components of adult fitness in Drosophila melanogaster. J Evol Biol Res 3: 79–86.
Killick SC, Carlsson AM, West SA, Little TJ . (2006). Testing the pluralist approach to sex: the influence of environment on synergistic interactions between mutation load and parasitism in Daphnia magna. J Evol Biol 19: 1603–1611.
Kondrashov AS . (1988). Deleterious mutations and the evolution of sexual reproduction. Nature 336: 435–440.
Kortet R, Niemelä PT, Vainikka A, Laakso J . (2012). Females prefer bold males; an analysis of boldness, mate choice, and bacterial resistance in the field cricket Gryllus integer. Ecol Parasitol Immunol 1: 1–6.
López P, Martín J . (2005). Female Iberian wall lizards prefer male scents that signal a better cell-mediated immune response. Biol Lett 1: 404–406.
Mallet MA, Bouchard JM, Kimber CM, Chippindale AK . (2011). Experimental mutation-accumulation on the X chromosome of Drosophila melanogaster reveals stronger selection on males than females. BMC Evol Biol 11: 156.
McGuigan K, Petfield D, Blows MW . (2011). Reducing mutation load through sexual selection on males. Evolution 65: 2816–2829.
McKean K, Nunney L . (2005). Bateman's principle and immunity: phenotypically plastic reproductive strategies predict changes in immunological sex differences. Evolution 59: 1510–1517.
Nystrand M, Dowling DK . (2014). Dose-dependent effects of an immune challenge at both ultimate and proximate levels in Drosophila melanogaster. J Evol Biol 27: 876–888.
Otto SP . (2009). The evolutionary enigma of sex. Am Nat 174: S1–S14.
Park AW, Jokela J, Michalakis Y . (2010). Parasites and deleterious mutations: interactions influencing the evolutionary maintenance of sex. J Evol Biol 23: 1013–1023.
Pischedda A, Chippindale A . (2005). Sex, mutation and fitness: asymmetric costs and routes to recovery through compensatory evolution. J Evol Biol 18: 1115–1122.
Punzalan D, Delcourt M, Rundle HD . (2013). Comparing the intersex genetic correlation for fitness across novel environments in the fruit fly, Drosophila serrata. Heredity 112: 143–148.
R Core Team. (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria.
Rantala MJ, Roff DA . (2007). Inbreeding and extreme outbreeding cause sex differences in immune defence and life history traits in Epirrita autumnata. Heredity 98: 329–336.
Restif O, Amos W . (2010). The evolution of sex-specific immune defences. Proc R Soc B 277: 2247–2255.
Roze D, Otto SP . (2011). Differential selection between the sexes and selection for sex. Evolution 66: 558–574.
Sharp NP, Agrawal AF . (2008). Mating density and the strength of sexual selection against deleterious alleles in Drosophila melanogaster. Evolution 62: 857–867.
Sharp NP, Agrawal AF . (2012a). Male-biased fitness effects of spontaneous mutations in Drosophila melanogaster. Evolution 67: 1189–1195.
Sharp NP, Agrawal AF . (2012b). Evidence for elevated mutation rates in low-quality genotypes. Proc Natl Acad Sci USA 109: 6142–6146.
Simmons LW, Roberts B . (2005). Bacterial immunity traded for sperm viability in male crickets. Science 309: 2031–2031.
Stoehr AM, Kokko H. . (2006). Sexual dimorphism in immunocompetence: what does life history theory predict? Behav Ecol 17: 751–756.
Vincent CM, Sharp NP . (2014). Sexual antagonism for resistance and tolerance to infection in Drosophila melanogaster. Proc R Soc B 281: 20140987.
West S, Lively C, Read A . (1999). A pluralist approach to sex and recombination. J Evol Biol 12: 1003–1012.
Whitlock MC, Agrawal AF . (2009). Purging the genome with sexual selection: reducing mutation load through selection on males. Evolution 63: 569–582.
Whitlock MC, Bourguet D . (2000). Factors affecting the genetic load in Drosophila: synergistic epistasis and correlations among fitness components. Evolution 54: 1654–1660.
Winterhalter WE, Fedorka KM . (2008). Sex-specific variation in the emphasis, inducibility and timing of the post-mating immune response in Drosophila melanogaster. Proc R Soc B 276: 1109–1117.
Young JA, Yourth CP, Agrawal AF . (2009). The effect of pathogens on selection against deleterious mutations in Drosophila melanogaster. J Evol Biol 22: 2125–2129.
Acknowledgements
We are grateful to D Guttman and his lab for providing Pseudomonas stocks, and to A Agrawal, R Baker and A Cutter for providing experimental materials and equipment. Thanks to G Apidianakis, C Diegel, J Kozlowska and K Schreiber, for advice, and to L St-Amant, H Kwok, M Nambakkam and F So for laboratory assistance. A Agrawal, L Holman, P Luijckx, A Wardlaw and anonymous reviewers provided helpful comments on previous versions of the manuscript. This work was supported by NSERC scholarships to NPS and CMV, and by Toronto Zoo IPS and L'Oreal-UNESCO scholarships to CMV.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sharp, N., Vincent, C. The effect of parasites on sex differences in selection. Heredity 114, 367–372 (2015). https://doi.org/10.1038/hdy.2014.110
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hdy.2014.110
This article is cited by
-
Investigating the interaction between inter-locus and intra-locus sexual conflict using hemiclonal analysis in Drosophila melanogaster
BMC Ecology and Evolution (2022)
-
Drosophila melanogaster hosts coevolving with Pseudomonas entomophila pathogen show sex-specific patterns of local adaptation
BMC Ecology and Evolution (2022)