Abstract
Maternally transmitted associations between endosymbiotic bacteria and insects are diverse and widespread in nature. Owing to imperfect vertical transmission, many heritable microbes have evolved compensational mechanisms to enhance their persistence in host lineages, such as manipulating host reproduction and conferring fitness benefits to host. Symbiont-mediated defense against natural enemies of hosts is increasingly recognized as an important mechanism by which endosymbionts enhance host fitness. Members of the genus Spiroplasma associated with distantly related Drosophila hosts are known to engage in either reproductive parasitism (i.e., male killing) or defense against natural enemies (the parasitic wasp Leptopilina heterotoma and a nematode). A male-killing strain of Spiroplasma (strain Melanogaster Sex Ratio Organism (MSRO)) co-occurs with Wolbachia (strain wMel) in certain wild populations of the model organism Drosophila melanogaster. We examined the effects of Spiroplasma MSRO and Wolbachia wMel on Drosophila survival against parasitism by two common wasps, Leptopilina heterotoma and Leptopilina boulardi, that differ in their host ranges and host evasion strategies. The results indicate that Spiroplasma MSRO prevents successful development of both wasps, and confers a small, albeit significant, increase in larva-to-adult survival of flies subjected to wasp attacks. We modeled the conditions under which defense can contribute to Spiroplasma persistence. Wolbachia also confers a weak, but significant, survival advantage to flies attacked by L. heterotoma. The host protective effects exhibited by Spiroplasma and Wolbachia are additive and may provide the conditions for such cotransmitted symbionts to become mutualists. Occurrence of Spiroplasma-mediated protection against distinct parasitoids in divergent Drosophila hosts suggests a general protection mechanism.
Similar content being viewed by others
Introduction
Associations between maternally transmitted endosymbiotic bacteria and insect hosts are pervasive and exert strong influence on their ecological and evolutionary dynamics (Moran et al., 2008). Some of these heritable symbioses are obligate, with host and symbiont completely dependent on each other for persistence (e.g., nutritional mutualisms; Douglas, 1998). Many other heritable symbionts are facultative, and thus, not absolutely required by the host for survival and reproduction (White et al., 2013). Approximately 40–66% of arthropod species are estimated to be infected with heritable facultative symbionts from a single bacterial genus (Wolbachia; Hilgenboecker et al., 2008; Zug and Hammerstein, 2012), but many more bacterial groups engage in such associations with insects (Moran et al., 2008). Vertical transmission of facultative symbionts is typically imperfect, and harboring the symbiont can be physiologically costly to the host. Consequently, heritable facultative symbionts can only persist in host populations, if they increase either the survival or production of infected female hosts (O’Neill et al., 1997). To ensure persistence, heritable facultative symbionts have adopted various strategies, namely, reproductive manipulation of their host (e.g., male-killing and cytoplasmic incompatibility (CI); Werren et al., 2008; Engelstadter and Hurst, 2009), and/or enhancement of host fitness through a diversity of mechanisms (Brownlie and Johnson, 2009; Ferrari and Vavre, 2011; Jaenike, 2012).
Several recent studies have reported facultative symbionts that confer protection to their host against parasites and pathogens (Hurst and Hutchence, 2010). Several bacterial symbionts of aphids confer protection against parasitoid wasps (Oliver et al., 2003, 2005; Vorburger et al., 2009) and fungi (Scarborough et al., 2005; Lukasik et al., 2012). Spiroplasma bacteria confer protection against fungi in the pea aphid (Lukasik et al., 2012), against a nematode in Drosophila neotestacea (Jaenike et al., 2010b) and against a parasitoid wasp in Drosophila hydei (Xie et al., 2010). Wolbachia has been shown to increase resistance or tolerance of Drosophila and mosquitoes against RNA viruses and against the protozoan parasite Plasmodium (Hedges et al., 2008; Teixeira et al., 2008; Moreira et al., 2009; Osborne et al., 2009; Bian et al., 2010; Frentiu et al., 2010; Zele et al., 2012).
There is growing evidence that endosymbionts can use more than one strategy to enhance their persistence. Indeed, the use of CI and protection against RNA viruses, by Wolbachia in dipterans (Hedges et al., 2008; Teixeira et al., 2008; Moreira et al., 2009; Osborne et al., 2009; Glaser and Meola, 2010; Walker et al., 2011), may explain the recent spread of Wolbachia in natural populations of D. melanogaster (Riegler et al., 2005; Nunes et al., 2008; Richardson et al., 2012), and makes Wolbachia a promising agent for the control of dengue (Iturbe-Ormaetxe et al., 2011; Walker et al., 2011), a human pathogen transmitted by mosquitoes. Similarly, Rickettsia bacteria associated with whiteflies (order Hemiptera) directly enhance host fitness and also bias sex ratio toward female offspring (Himler et al., 2011). The fitness of Drosophila innubila infected with a male-killing Wolbachia strain is enhanced by both male-killing-dependent (i.e., resource reallocation due to death of male siblings) and male-killing-independent mechanisms (i.e., enhanced fecundity of nutrient-deprived hosts and increased survival to RNA virus infection; Unckless and Jaenike, 2012). Not all reproductive parasites examined to date, however, confer protection against natural enemies (e.g., the male-killing Wolbachia strain of Drosophila bisfasciata does not confer protection against RNA viruses; Longdon et al., 2012).
In Drosophila, the two defensive Spiroplasma strains known do not appear to engage in reproductive manipulation (Ota et al., 1979; Jaenike et al., 2010a), but several of their close relatives are male killers. One of these male-killing strains is the Melanogaster Sex Ratio Organism (hereafter MSRO), which can co-occur with Wolbachia in certain populations of D. melanogaster. When present, infection frequencies of Spiroplasma MSRO in wild populations of D. melanogaster range within 1.1–17% (Montenegro et al., 2005; Ventura et al., 2012). It is unclear whether direct or indirect fitness effects of male-killing are sufficient to maintain such infection frequencies, particularly those at the higher end. Martins et al. (2010) found that MSRO-infected wild females have a higher fecundity (at least over four consecutive days), and their progeny develop faster. In contrast, Montenegro et al. (2006) reported no effect of MSRO on larval competitive ability or adult fecundity of D. melanogaster Canton-S strain. It is possible that other fitness effects unrelated to its male-killing ability contribute to the prevalence of MSRO in nature. The work presented herein examines whether the male-killing Spiroplasma strain of D. melanogaster (MSRO) confers protection against parasitoid wasps. We also examine whether wMel, the Wolbachia strain known to cause CI and protect against RNA viruses, influences the outcome of the fly–parasitoid interaction.
Co-occurrence of two cytoplasmically transmitted symbionts may lead to cooperation or antagonism between them. On the basis of the nonrandom positive association of Wolbachia and Spiroplasma observed in D. neotestacea populations, Jaenike et al. (2010a) suggest that mutualism between the two symbionts might have evolved, but evidence for a cooperation mechanism itself has not been found. In contrast to D. neotestacea, no evidence for significant associations between the two symbionts has been observed in natural populations of D. melanogaster (Ventura et al., 2012). In addition, Montenegro et al. (2006) found no evidence of cooperation between the two endosymbionts in D. melanogaster, based on several lab-based fitness measures. Two instances of antagonism between make-killing Spiroplasma and Wolbachia have been observed. Spiroplasma densities negatively affect Wolbachia densities in D. melanogaster (Goto et al., 2006), but not vice versa (Goto et al., 2006; Silva et al., 2012). In addition, Silva et al. (2012) found that the male-killing ability of Spiroplasma MSRO was stronger in the absence of Wolbachia. Other cases of conflict or cooperation, however, may be revealed under conditions not tested to date, such as in the defense against natural enemies. Therefore, our study also examines if the outcome of a parasitoid wasp attack is influenced by co-occurrence of Wolbachia and Spiroplasma.
The specificity of the symbiont-mediated protection against natural enemies will influence the ecological and evolutionary dynamics of the host and its protective symbiont. Numerous species of parasitoid wasps attack Drosophila flies (Fleury et al., 2009). D. melanogaster alone is an adequate host to at least 14 species from four families of parasitic Hymenoptera that use diverse strategies to circumvent host defenses (Kacsoh and Schlenke, 2012). Our study examined whether Spiroplasma and Wolbachia influence the outcome of parasitism by two cosmopolitan congeneric wasps that differ in their host range and attack strategies: the Drosophila generalist Leptopilina heterotoma (Lh) and the melanogaster-group specialist L. boulardi (Lb). Although Lb causes partial suppression of host defenses, it tends to evade passively host immunity by embedding its eggs within host tissues, thereby avoiding encapsulation by host lamellocytes. In contrast, the eggs of Lh, which float freely in the host hemocoel, avoid encapsulation via a more aggressive suppression of host defenses, including the destruction of lamellocytes (Lee et al., 2009). Therefore, knowledge on the parasitoid species against which Spiroplasma confers protection will provide insight into the generality of the protection and the possible defensive mechanism(s).
This work expands our knowledge on defensive associations of Drosophila in general, and of the model organism D. melanogaster in particular, by revealing that: (a) as reported for its non-male-killing counterpart, a male-killing Spiroplasma strain is capable of protecting its host against wasp-induced mortality, by slowing down wasp larval growth and preventing successful wasp development; (b) although the observed degree of protection alone might not guarantee Spiroplasma prevalence in nature, it may be relevant to persistence in combination with the fitness advantages derived from its male-killing ability; (c) this protection is conferred against two species of wasps with contrasting strategies, suggesting a general defensive mechanism; and (d) the positive additive effect of Wolbachia and Spiroplasma on fly survival against attack by at least one species of wasp provides empirical evidence of a mechanism by which two cytoplasmically transmitted endosymbionts could become mutualists.
Materials and methods
Insect sources
Seven isofemale lines (hereafter fly isolines) were established from mated wild-caught D. melanogaster females collected with orange baits in Tapachula, Chiapas, Mexico, in January 2011. To identify potential heritable endosymbionts of these flies, at least three females per isoline were subjected to sterile ovary dissection and DNA extraction as described in Mateos et al. (2006). Three sets of universal polymerase chain reaction (PCR) primer screenings were then conducted on the DNA extracts: (1) primers for bacterial 16S rRNA gene (10F–1507R); (2) primers for bacterial 16S rRNA gene (27F–1495R); and (3) primers for 16S–23S rRNA gene fragment (559F–35R). In addition, screening with Wolbachia- and Spiroplasma-specific PCR primers was conducted (primers and conditions described in Xie et al., 2010). These results indicated that all seven isofemale lines were infected with Wolbachia wMel, but not with any other heritable endosymbionts.
For the generalist wasp Lh, we used the highly virulent inbred strain Lh14, which is infected with Wolbachia (Schlenke et al., 2007). For the specialist wasp Lb, we used the highly virulent inbred strain Lb17. This wasp strain lacks infection by Wolbachia (Schlenke et al., 2007) and by the Lb filamentous virus (Gueguen et al., 2011), a virus linked to superparasitism behavior in this species (Varaldi et al., 2003, 2006). Wasps were maintained in D. melanogaster Canton-S with standard cornmeal food.
Generation of endosymbiont treatments
For each of the seven original fly isolines, we generated four endosymbiont treatments: uninfected (S–W–); infected with Wolbachia wMel only (S–W+); infected with Spiroplasma MSRO only (S+W–); and doubly infected (S+W+) (see Supplementary Figure S1). To generate the Wolbachia-free (W–) treatments, a subset of each isoline was treated for three consecutive generations with a combination of tetracycline and erythromycin (added to the food at a final concentration of 0.2 and 0.16 mg ml; respectively). The Wolbachia-specific PCR screening described above confirmed removal of Wolbachia. In an effort to restore their regular microbiota, flies eclosing from the antibiotic treatment were temporarily placed in vials that had previously housed untreated flies, and maintained on antibiotic-free food for three consecutive generations. A subset of the resulting 14 fly lines, seven lacking Wolbachia (W–) and seven infected with Wolbachia (W+), were then artificially infected with Spiroplasma MSRO via adult-to-adult hemolymph transfer as described in Xie et al. (2010). The donor flies were naturally infected with Spiroplasma MSRO, and were originally collected in Campinas, São Paulo State, Brazil (1997) and maintained in the lab by crossing to Canton-S males (Montenegro et al., 2000). Success of artificial infection and establishment of vertical transmission of Spiroplasma was confirmed by all-female progeny and PCR screenings with Spiroplasma-specific primers over at least three subsequent generations.
Fly survival assay
This experiment was carried out at least four generations after Spiroplasma artificial infection. Before experiments, all the flies were maintained at low-density larval conditions. For each isoline and endosymbiont treatment (7 isolines × 4 endosymbiont treatments=28), we conducted approximately three replicates (28 × 3=84 replicates). Each replicate consisted of a mating/oviposition group (three females plus six males). Females were <15 days old; males were from the same isoline and Wolbachia infection status as females, but free of Spiroplasma. Mating groups were allowed to mate and oviposit on standard cornmeal vials for 2 days, after which they were transferred to a fresh food vial. Approximately 30 first/second instar larvae (2 days old) per vial were collected and transferred into a fresh vial. Three larvae vials were generated per replicate (approximately 84 × 3=252 larvae vials; see Supplementary Figure S1). Each vial per replicate was subjected to one of the following wasp treatments: (1) no wasp control; (2) Lh; or (3) Lb. Five ∼3-day-old wasps were added per vial and allowed to oviposit for 2 days. For each vial, we recorded the number of starting fly larvae, puparia, emerging flies and emerging wasps. Endosymbiont infection status of the three mothers used in each replicate was examined by the Wolbachia- and Spiroplasma-specific PCR assays described above. Only replicates for which all three mothers had the expected infection status were used in the analyses. In addition, to assess Spiroplasma MSRO vertical transmission rate in the presence and absence of Wolbachia, we used PCR to examine the Spiroplasma infection status of 10 female flies per replicate per isoline emerging from the treatments lacking wasps (approximately=140 total).
We used SAS Enterprise Guide version 4.2 statistical package (SAS Institute Inc., Cary, NC, USA) to fit a generalized linear mixed model with a binomial distribution of the raw data for: (a) number of emerging adult flies/initial number of fly larvae (i.e., fly larva-to-adult survival rate); (b) number of emerging adult flies/total number of puparia (i.e., fly pupa-to-adult survival rate); (c) number of pupae/initial number of fly larvae (i.e., fly larva-to-pupa survival rate); (d) number of emerging adult wasps/initial number of fly larvae; and (e) number of emerging adult wasp/total number of puparia. The independent variables were Spiroplasma infection status (fixed), Wolbachia infection status (fixed) and their interaction term (fixed), fly strain (isoline, random). The random interactions (i.e., isoline × Wolbachia, isoline × Spiroplasma, isoline × Wolbachia × Spiroplasma) were excluded from final model owing to the lack of significance. Significance tests of random effects were based on the ratio of pseudo-likelihoods (Covtest in SAS).
Differential oviposition and development of parasitoids in D. melanogaster
To examine whether wasps lay different number of eggs in fly larvae with different endosymbiont infections, we compared the number of wasp eggs or larvae per fly larva among the four endosymbiont infection treatments. In addition, to examine whether Spiroplasma MSRO and/or Wolbachia wMel affect the larval growth rate of Lh and Lb in D. melanogaster, we measured wasp body length in the four endosymbiont infection treatments at several time points. These assays were conducted separately from the fly fitness experiments on three out of the seven isolines. We followed the same protocol described above to set up mating groups, collect larvae and apply the wasp treatments, except that the no-wasp control was omitted. Immediately after wasp removal (hereafter time point 0 h), 10 fly larvae were collected per vial, and dissected under a microscope to count and measure wasp eggs/larvae. To examine wasp growth, we measured body length of the dominant wasp larva in each of five fly larvae per vial at one subsequent time point (72 h) for Lh, and at two subsequent time points (72 and 144 h) for Lb (only one subsequent time point was necessary to detect differences between endosymbiont treatments in Lh; see Results section). The dominant wasp larva in each fly larva was fixed in ∼96% ethanol and immediately digitally photographed with a stage micrometer. The software Spot Basic (version 4.7; Diagnostic Instruments, Sterling Heights, MI, USA) was used to measure body length as the straightline distance between the tip of the mouth and caudal end.
For the differential oviposition assay, we examined 20–40 fly larvae (10 larvae per vial) per treatment per fly isoline; each fly larva was treated as a replicate. We used SAS Enterprise Guide version 4.2 statistical package to fit a generalized linear mixed model with: (a) a binary distribution of the raw data for at least 1 vs 0 wasp eggs or larvae per fly larva; and (b) a Poisson distribution of the raw data for the number of the wasp eggs or larvae per fly larva. The independent variables were Spiroplasma infection status (fixed), Wolbachia infection status (fixed), and their interaction term (fixed), fly strain (isoline, random) and vial (random, nested within isoline). Significance tests of random effects were based on the ratio of pseudo-likelihoods (Covtest in SAS).
For the wasp development assay, we performed at least three replicates per treatment per fly isoline; each replicate corresponded to a measurement of the dominant wasp egg/larva in a single fly larva. We used SAS Enterprise Guide version 4.2 statistical package to fit a general linear mixed model with the raw measurement of wasp body length. The independent variables were Spiroplasma infection status (fixed), Wolbachia infection status (fixed), hours after wasp attack (fixed) and all of their interaction terms (fixed), and fly strain (isoline, random). Nonsignificant interactions were excluded from the final analysis. Significance tests of random effects were based on the ratio of pseudo-likelihoods (Covtest in SAS).
Results
Fly survival and wasp success
The data generated in this study have been deposited in Dryad under accession numbers doi: 10.5061/dryad.47574. In the absence of parasitoid wasps, mean fly larva-to-adult survival was >87.85% in all the endosymbiont infection treatments (Figure 1a). Neither Spiroplasma nor Wolbachia infection states were significant for any of the fly survival measures. The effect of fly isoline, however, was significant for both larva-to-pupa survival (χ2=5.72, P=0.0084; Figure 1a and Supplementary Table S1) and pupa-to-adult survival (χ2=2.87, P=0.0451; Figure 1a and Supplementary Table S1), but not for larva-to-adult survival (χ2=0.59, P=0.221; Supplementary Table S1). The effect of isoline was not significant for any of the survival measures in any of the wasp treatments (Supplementary Table S1), and is thus not discussed any further.
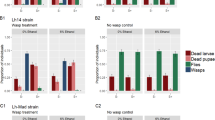
Fly larva-to-adult survival, larva-to-pupa survival, pupal mortality and wasp success in the four endosymbiont infection treatments (S=Spiroplasma; W=Wolbachia) and in the three wasp treatments. (a) No wasp control. (b) Lh treatment. (c) Lb treatment. P-values shown for each effect: Spiroplasma infection state; Wolbachia infection state; their interaction; and fly strain (isoline). For isoline, only significant P-values are shown (see Supplementary Table S1). Bars: white, proportion of fly larvae that survived to adulthood; gray, proportion of fly larvae that survived to pupation; black, proportion of total pupae that failed (neither fly nor wasp emerged); dotted, exposed fly larvae that gave rise to eclosing wasps. Error bars: s.e.
In the presence of the generalist wasp Lh, Spiroplasma infection had a significantly positive effect on fly larva-to-adult survival and on pupa-to-adult survival (respectively, F1,84=6.72, P=0.0041 in Figure 1b and F1,84=9.34, P=0.003 in Supplementary Table S1). Similarly, Wolbachia infection also had a significantly positive effect on these two measures (F1,84=5.16, P=0.0256 in Figure 1b and F1,84=4.58, P=0.0353 in Supplementary Table S1). The interaction between Spiroplasma and Wolbachia was not significant. The positive effect of each symbiont on fly survival was small and appears to be additive or slightly synergistic; mean larva-to-adult survivorship of the four endosymbiont treatments was: endosymbiont-free (S–W–)=0.86%; Wolbachia-infected (S–W+)=2.59%; Spiroplasma-infected (S+W–)=3.28%; and doubly infected (S+W+)=7.78% (Supplementary Table S1).
Spiroplasma had a strong and highly significant (F1,84=196.39, P<0.0001; Figure 1b and Supplementary Table S1) negative effect on the success of Lh, measured as the proportion of exposed fly larvae that gave rise to eclosing wasps: Spiroplasma-infected means were 3.63% (S+W–) and 0.9% (S+W+), whereas Spiroplama-free means were 80.03% (S–W–) and 72.09% (S–W+). Wolbachia appears to reduce Lh wasp success slightly, albeit significantly (F1,84=6.42, P=0.013; Figure 1b and Supplementary Table S1). In essence, a large proportion (∼89–92%) of pupae failed to complete development in the Spiroplasma-infected Lh-attacked treatments but not in the absence of Spiroplasma (∼5–13%) or in the absence of wasps (∼3–6%; Figure 1 and Supplementary Table S1). These results suggest that although Spiroplasma may not be highly efficient at rescuing the flies from a wasp attack, it is efficient at preventing wasp success. The effects of either symbiont were only detectable in measures encompassing the pupa-to-adult stage. In contrast, larva-to-pupa survival was relatively high and not significantly different among endosymbiont treatments (range=82.51–85.66%; Figure 1b and Supplementary Table S1).
In the presence of the specialist parasitoid wasp Lb, the effect of Spiroplasma, but not of Wolbachia, on fly survival and wasp success was similar to that observed in the presence of Lh. Spiroplasma significantly enhanced fly larva-to-adult survival (F1,87=7.29, P=0.0083; Figure 1c) and pupa-to-adult survival (F1,87=9.26, P=0.0031; Supplementary Table S1). In contrast, although the means suggest a potentially positive effect of Wolbachia on fly survival (Figure 1c), this effect was not significant for any of the fly survival measures. As in the Lh treatment, the effect of Spiroplasma on fly fitness in the Lb treatment was only detectable in measures involving the pupa-to-adult stage. Despite the significant effect of Spiroplasma on fly fitness, the fitness benefit from Spiroplasma infection is small (mean larva-to-adult survival: S–W–=1.26%; S–W+=2.16%; S+W–=3.13%; and S+W+=6.91%; Supplementary Table S1). Nevertheless, wasp success in the presence of Spiroplasma was extremely low (mean S+W+=1.89%; mean S+W–=3.15%) and significantly different from the treatments lacking Spiroplasma (mean S–W–=73.6%; mean S–W+=72.95%). As with Lh, the main outcome of Spiroplasma infection in the Lb treatments was failed pupae, which contrasts with the relatively high success of both wasp species in the absence of Spiroplasma.
The higher fly survival observed in S+ treatments (which were all-female) could be due to a higher host-encoded resistance of female flies against Leptopilina wasps, rather than Spiroplasma-encoded protection. Indeed, a study by Kraaijeveld et al. (2008) found that Drosophila males are less likely than females to encapsulate an egg from the braconid wasp Asobara tabida. We therefore tested for an effect of Leptopilina treatment on host sex ratio in treatments lacking male-killing Spiroplasma: D. melanogaster with and without Wolbachia (S–W+ and S–W–, respectively) and D. hydei with and without a non-male-killing strain of Spiroplasma (S+W– and S–W–, respectively) that confers protection against Lh (Xie et al., 2010). The effect of Leptopilina on host sex ratio (proportion of surviving male flies) was not significant (see Supplementary Table S4 and Supplementary Figure S2 for results and details). These results indicate that the small survival advantage observed in Spiroplasma-infected flies against Leptopilina wasps is unlikely a result of superior female resistance or tolerance. Nevertheless, this possibility cannot be completely ruled out, due to a limited power stemming from the extremely low number of surviving individuals in the Spiroplasma-free treatments subjected to wasps.
The overall vertical transmission rate of Spiroplasma MSRO was 97% in this experiment. Spiroplasma MSRO vertical transmission rate was not significantly different between Wolbachia-infected and uninfected flies (95% and 99%, respectively; F1,14=1.62, P=0.2244).
Differential oviposition
Several observations suggest that the presence of Spiroplasma prevents successful development of the two wasp species upon oviposition: (a) extremely low wasp emergence in the presence of Spiroplasma; (b) large proportion of failed pupae not observed in the absence of wasps; and (c) the presence of a detectable effect of Spiroplasma on fly survival only at the pupa-to-adult stage, which is consistent with the stage at which protection by Spiroplasma hy1 is detectable in D. hydei attacked by Lh (Xie et al., 2010). Nevertheless, a preoviposition mechanism may have contributed to the low degree of wasp emergence observed (e.g., if female wasps were able to detect Spiroplasma infection and preferred to oviposit on Spiroplasma-free fly larvae). We therefore examined whether the two species of wasps lay different numbers of eggs according to the endosymbiont infection status of the fly larvae, under equivalent conditions to the fitness assays described above. Wasps were not given a choice of infected and uninfected fly larvae. The number of wasp eggs found per fly larva did not differ significantly among different Spiroplasma and Wolbachia infection states for either the generalized linear mixed model with Poisson distribution or the generalized linear mixed model with a binary distribution (i.e., one or more wasp eggs grouped into a single category; Figure 2 and Table 1). A significant difference was observed however, between the two wasp species, regarding the exact number of wasp eggs per fly larva. Lb females tended to lay more eggs per host larva (mean±s.e.=3.69±0.2 wasp eggs, among all the parasitized fly larvae and pooled across endosymbiont treatments) than Lh females (mean±s.e.=2.10±0.12), regardless of the fly endosymbiont infection states (F1,287=16.35, P<0.0001). The superparasitism rate (i.e., number of fly larvae with two or more wasp eggs/number of parasitized fly larvae) was 83.47% in Lb and 52.76% in Lh treatment. Although this observation contrasts with the report by Gueguen et al. (2011) that the same wasp strain (Lb17) does not superparasitize, the difference may be explained by the higher parasitism pressure of our assay; five female wasps competing for ∼30 fly larvae over 48 h in this study vs one female wasp exposed to 10 fly larvae over 17 h in Patot et al. (2009) and Gueguen et al. (2011). The average oviposition rate (i.e., proportion of fly larvae with at least one wasp egg or larva) was 87.17% for Lh and 90.98% for Lb. These results suggest that although a preoviposition mechanism does not appear to explain the differential survival of flies with and without Spiroplasma, the few flies emerging from the wasp treatments might have not been attacked.
Wasp growth rate
The presence of Spiroplasma, but not of Wolbachia, interfered with normal larval growth of both wasp species. The two species of wasps started out at similar body lengths (∼0.33 mm; 0 h), hatched successfully (at least the dominant wasp larva when more than one wasp egg was present), and achieved some initial growth (Figure 3). Spiroplasma infection state, hours after attack and their interaction had a highly significant effect on the body length of both wasp species (see Table 2). The significant Spiroplasma infection state and hours after attack interaction indicates that wasp growth rate differs between the Spiroplasma-infected and -uninfected treatments (Figures 3a and b). Lb and Lh differed, however, in the time point and wasp length at which a significant decrease in wasp growth rate was detectable: 72 h for Lh and 144 h for Lb (Table 2).
Conditions under which defense against wasps may contribute to Spiroplasma MSRO persistence
The equilibrium prevalence of a male-killing endosymbiont depends on the advantage that females gain by the infection, the viability and fertility cost of infection to females and the transmission efficiency (Dyer and Jaenike, 2004). Dyer and Jaenike (2004) developed a model in which the fitness of female progeny produced by an infected female is equal, regardless of their infection status (i.e., uninfected females benefit just as much as their infected sisters from the symbiont-induced death of their infected brothers). To assess the conditions under which the Spiroplasma-induced defense observed in our study might contribute to persistence, we modified the model of Dyer and Jaenike (2004) to account for the unequal fitness of uninfected and infected progeny produced by the same infected mother.
Under the assumption of constant parasitoid attack, let the fitness of a Spiroplasma-infected female be 1 and that of an uninfected female be 1−s, where s is the fitness difference due to the Spiroplasma infection and β is the proportion of infected daughters produced by the infected mother (vertical transmission efficiency). If I is the prevalence of infection among females in one generation, then their daughter’s generation infection prevalence (I′) is

Equation (1) has two equilibria. When I=0, there is no Spiroplasma infection in the host population. Hurst (1991) modeled the invasion of a male killer under the resource release hypothesis; thus, this equilibrium will not be discussed further here. The other equilibrium is reached when I=I′,

At this internal equilibrium for Equation (1), the fitness difference between Spiroplasma-infected and -uninfected flies is:

When β=0.97 and I ranges between ∼1 and 17.7% (i.e., the range of Spiroplasma prevalence observed in D. melanogaster natural populations), s must range between ∼0.0303 and 0.03622 to maintain the equilibrium frequency I (Equation (1)).
Now, assuming that Spiroplasma-infected and -uninfected females undergo equal wasp attack rates (as suggested by our oviposition assay), as well as equal mortality rates in the absence of wasps (as suggested by the survival assay), the relative fitness of Spiroplasma-infected to uninfected flies according to the survival assay of the present study is:

According to the Spiroplasma-enhanced larva-to-adult survival observed in our experiments, s=0.33–0.94 in the presence of Lh and s=0.14–0.87 in the presence of Lb (FitnessUn=S–W– and FitnessIn=S+W– values from mean±s.e. of larva-to-adult fly survival from Supplementary Table S1; details for calculation of s ranges in Supplementary Table S2). These values are largely above those required to observe equilibrium frequencies of ∼1–17.7%. These findings suggest that, in the context of high wasp parasitism (100%), defense against wasps could have a major role in the persistence of the male-killing Spiroplasma strain of D. melanogaster.
Nevertheless, although wasp parasitism rates can be high in nature, they are unlikely to be 100%, and they vary over time and space (reviewed in Fleury et al., 2009). If we take into account imperfect parasitism rate (P), and define the fitness of unattacked flies as 1 (regardless of the Spiroplasma infection), and the post-wasp attack fitness of Spiroplasma-infected and -uninfected flies as k and h, respectively, then, at equilibrium I′:

As above, the equilibrium I=0 will not be discussed. For the internal equilibrium I′= I, β>0; 0<P⩽1 and 0<k<1, thus Pk+1−P≠0, and:

Here, the fly survival rate observed in the absence of wasps (mean of all four endosymbiont treatments=89.5%) is assumed to represent the fitness of unattacked flies and used to standardize the k and h observed in this study for each wasp species assay. We also assume that most of the surviving flies within the wasp treatments were indeed attacked by wasps (i.e., ∼87% for Lh and ∼91% for Lb treatment, based on our observed oviposition rates). The relationship of wasp parasitism rate (P) to Spiroplasma prevalence (I) for both wasp species is shown in Figure 4. Under these conditions, Lh parasitism rate P must be >53.92% and >58.31% to maintain a Spiroplasma equilibrium frequency (I) of 1% and 17.7%, respectively (solid line; Figure 4a and Supplementary Table S3). For Lb, P must be >60.43% and >64.65%, respectively, to maintain comparable Spiroplasma equilibrium frequencies (solid line; Figure 4b and Supplementary Table S3).
Relationship of wasp parasitism rate (P) to Spiroplasma prevalence (I) in the fly population for (a) Lh and (b) Lb, according to the larva-to-adult survival advantage conferred by Spiroplasma MSRO, as estimated directly from our experiments (solid line), and an adjusted fitness advantage accounting for reduced longevity and fecundity of adult flies surviving a wasp attack (dashed line; see text for details). Gray areas indicate the range of prevalences (1–17.7%) reported for Spiroplasma MSRO in natural populations of D. melanogaster.
The post-wasp attack reproductive fitness of Spiroplasma-infected flies (k), however, may be lower than that observed in this study, as Xie et al. (2011) showed that Spiroplasma-infected flies (D. hydei) surviving a wasp attack (Lh) suffer detrimental fitness effects after eclosion (i.e., ∼34% reduction in adult 0- to 10-day longevity and ∼30% reduction in fecundity). To account for a potentially equivalent fitness decrease after eclosion in Spiroplasma-infected D. melanogaster, we also examined the relationship between Spiroplasma prevalence (I) and wasp parasitism rate (P), under a more conservative value for k (i.e., observed k × 0.66 × 0.7). Under this lower k, Lh parasitism rate P must be >81.69% and >84.30%, respectively, to maintain a Spiroplasma equilibrium frequency of 1% and 17.7% (dashed line; Figure 4a). Even higher levels of Lb parasitism are required to maintain comparable Spiroplasma equilibrium frequencies; P must be >94.96% and 95.95% for I=1% and 17.7%, respectively (dashed line; Figure 4b).
Discussion
The present work indicates that Spiroplasma MSRO, a maternally transmitted reproductive parasite of D. melanogaster, prevents successful development of two parasitoid wasps (Lh and Lb). These results expand the taxonomic diversity of Spiroplasma-mediated parasitoid killing from D. hydei to D. melanogaster (two species that diverged up to ∼63 million years ago; Tamura et al., 2004), from the non-male-killing strain hy1 (Xie et al., 2010) to its male-killing relative MSRO (divergent by ∼1.8% at the fru locus; uncorrected p-distance; GenBank accession nos. AJ628444 and FJ657017), and from Lh to its congeneric, but distant relative Lb (∼14% divergent at the cytochrome oxidase I gene; uncorrected p-distance; GenBank accession nos. JQ808444 and JQ808436).
Can the defense against wasps contribute to the persistence of male-killing Spiroplasma?
The results suggest that Spiroplasma MSRO confers a small, albeit significant, survival advantage to flies that have been attacked by either species of wasp. Fly survival against Lh was approximately 3.8 times higher in the S+W– treatment (mean=3.28%) than in S–W– treatment (mean=0.86%). Similarly, fly survival against Lb was approximately 2.5 times higher in the S+W– treatment (mean=2.15%) than in S–W– treatment (mean=0.86%). The above advantage conferred by Spiroplasma contrasts with that reported for D. hydei attacked by Lh, where Spiroplasma hy1 increases larva-to-adult survival approximately 9.25 times; from ∼4% in the S– treatment to ∼37% in the S+ treatment (Xie et al., 2010). The small selective advantage conferred by Spiroplasma MSRO in the present study raises the question as to whether this protective mechanism is relevant to Spiroplasma persistence.
To address the above question, we developed a model that takes into account vertical transmission efficiency and the selective advantage of infection (s) under conditions of high wasp parasitism (see Results). Under such conditions, and based on our experimentally determined vertical transmission rates and larva-to-adult survival advantage, Spiroplasma MSRO is expected to persist at the range of infection frequencies observed in nature (∼1–17.7%). We then modified the model to account for lower and more realistic wasp parasitism rates. In addition, we assumed a lower post-wasp attack fitness of Spiroplasma-infected flies (k) to account for the reported reduction in adult fecundity and longevity experienced by D. hydei surviving a parasitoid attack (Xie et al., 2011). These results suggest that maintenance of Spiroplasma at infection frequencies observed in nature can only be achieved at wasp parasitism rates >82% for Lh and >95% for Lb. Although up to 80% parasitized Drosophila larvae have been reported in several regions, an average parasitism range of 5–40% is more common, which fluctuates geographically and seasonally (reviewed in Fleury et al., 2009). Therefore, it appears that the selective advantage conferred by defense alone does not guarantee Spiroplasma persistence. Nevertheless, it is possible that a combination of defense and other net fitness benefits conferred by this male-killing strain (i.e., higher fecundity of wild-caught flies and faster development; Martins et al., 2010) ensure its persistence. Furthermore, our experiment was limited to a few host backgrounds (seven isofemale lines not known to harbor Spiroplasma naturally), and two highly virulent wasp strains. It is possible that combinations of other host and wasp backgrounds present in nature result in more (or less) efficient rescue by Spiroplasma.
Effect of Wolbachia wMel and its co-occurrence with Spiroplasma MSRO on the outcome of wasp parasitism
Wolbachia wMel had a weak positive, but nonsignificant, effect on survival of flies subjected to Lb attack. Lack of a significant effect of wMel on the interaction of Lb with D. melanogaster (two backgrounds) was also reported by Martinez et al. (2012). Other strains of Wolbachia are reported to have negative and positive effects on the interaction of D. simulans with Lb (Fytrou et al., 2006; Martinez et al., 2012), but these effects are dependent on whether or not Lb carries the virus LbFV (Martinez et al., 2012), which does not occur in the Lb strain used in our study (Gueguen et al., 2011). Thus, it appears that in D. melanogaster, at least, Wolbachia wMel does not significantly influence the outcome of oviposition by Lb.
Infection with Wolbachia wMel significantly reduced parasitism success of Lh, but its effect was much smaller than that of Spiroplasma MSRO. Fly survival against Lh attack was also significantly enhanced by wMel at a similar rate as Spiroplasma MSRO (S–W+ mean=2.6% vs S+W– mean=3.3%). The effect of the two symbionts on fly survival appears to be additive (S+W+ mean=7.8%). These observations provide empirical evidence for a mechanism by which two cytoplasmically transmitted endosymbionts may evolve cooperation. If the observed additive benefits of coinfection by Spiroplasma and Wolbachia against Lh are ecologically relevant, we expect a nonrandom positive association of the two symbionts in natural populations of D. melanogaster, such as that observed in D. neotestacea (Jaenike et al., 2010a). Nonetheless, Ventura et al. (2012) failed to detect a significant association between the two symbionts in natural populations of D. melanogaster in Brazil. Therefore, it is possible that the additive effects observed in our lab experiments are too weak to counter potential disadvantages of coinfection in nature, including the antagonistic reproductive manipulation strategies of the two symbionts: the CI of Wolbachia, which relies on infected males vs the male-killing effect of Spiroplasma.
Wasp-killing mechanism
The extremely low success of wasps in the presence of Spiroplasma MSRO could be the result of reduced oviposition rates (i.e., a preoviposition mechanism), or reduced survival of developing wasps in Spiroplasma-infected flies (i.e., a postoviposition mechanism). Our wasp oviposition results indicate that wasps do not lay significantly different numbers of eggs in any of the four endosymbiont treatments, ruling out a pre-oviposition mechanism. Furthermore, the high proportion of dead pupae observed only when both Spiroplasma and wasps were present provides additional evidence that wasp failure associated with the presence of Spiroplasma is exerted mostly at the pupa-to-adult stage, and thus, after oviposition.
The mechanism by which Wolbachia wMel appears to enhance fly survival of Lh-attacked flies is unclear. The wasp oviposition results suggest that it occurs after oviposition, but wasp growth rates are not affected by wMel. Wolbachia wMel has been reported to increase hemolymph melanization in D. melanogaster (Thomas et al., 2011), but evidence for melanization was not observed in Lh-attacked flies (discussed below).
Both wasp species exhibited slower larval growth rates in D. melanogaster infected with Spiroplasma MSRO, but wMel had no effect on wasp growth rate. Slower growth was also reported in Lh developing within D. hydei infected with Spiroplasma hy1 (Xie et al., 2011). Within D. melanogaster, although the growth trajectory of the two wasps in the hosts lacking Spiroplasma is similar, the growth inhibition mediated by Spiroplasma MSRO is detectable earlier in Lh than in Lb. The differences between the two wasps may reflect different interactions between the fly, wasp and endosymbiont, including the possible effect of Lb superparasitism (e.g., injection of larger venom amounts through repeated oviposition may counter the effects of Spiroplasma). For example, the parasitoid wasp Aphidius ervi intentionally superparasitizes endosymbiont-infected aphids, presumably to overcome the symbiont-encoded defense (Oliver et al., 2012). In our study, however, the higher superparasitism of Lb compared with Lh does not seem to result in higher wasp survival.
One of the mechanisms by which Spiroplasma could cause wasp death is by enhancing host immunity (e.g., melanotic encapsulation). Lh counters host defenses by destroying lamellocytes, one of the essential cell types responsible for encapsulation (Morales et al., 2005; Lee et al., 2009). Our results with Lh suggest that Spiroplasma does not enhance this aspect of fly immunity, as we observed no melanized tissues in any Lh-attacked flies at the time point examined (i.e., 72 h after attack; not shown), and all the wasp embryos hatched successfully. Lack of melanization was also reported in D. hydei attacked by Lh, regardless of Spiroplasma infection state (Xie et al., 2011).
In contrast to Lh, the strategy of Lb includes embedding embryos within host tissues and altering lamellocyte shape without causing lamellocyte lysis (Lee et al., 2009). As a result, encapsulation is thwarted, but subsequent melanization and systemic production of antimicrobial peptide production continue (Lee et al., 2009). In this study, some of the fly larvae in the Lb treatment exhibited melanized tissues at 72 and 144 h after attack. To be effective, however, melanotic encapsulation should kill the wasp before egg hatching, and it is typically completed by 24–40 h after attack (equivalent to ∼0 h in our study) (Russo et al., 1996; Williams et al., 2006). These observations suggest that Spiroplasma does not enhance the fly’s ability to encapsulate wasp embryos, but improvement of other aspects of immunity cannot be ruled out (e.g., enhancement of cytotoxic products such as reactive oxygen species or intermediates of the melanization cascade; Lemaitre and Hoffmann, 2007).
Two mechanisms unrelated to host-encoded immunity by which Spiroplasma may prevent wasp success include: the presence of a substance toxic to the developing wasp, and the absence (or reduction) of a substance necessary for wasp development. Although our results do not allow us to distinguish between these, observation of similar effects of two Spiroplasma strains (MSRO and hy1; poulsonii clade), in two distantly related Drosophila hosts (D. hydei and D. melanogaster) against two congeneric but distantly related parasitoid wasps (Lh and Lb), suggests that the mechanism might be quite general. Furthermore, the mycophagous fly D. neotestacea harbors a non-male-killing Spiroplasma strain (also within the poulsonii clade) that inhibits growth of Howardula aoronymphium, a parasitic nematode of adult hemocoel (Jaenike et al., 2010b). Thus, assuming the same mechanism is responsible for growth inhibition of the two types of endo-macroparasites (i.e., wasps and nematodes), this trait may have been present in the ancestor of the poulsonii clade, which includes male-killing and non-male-killing strains associated with several other species of Drosophila (e.g., D. nebulosa, D. willistoni and D. simulans; Haselkorn et al., 2009).
The present study indicates that Spiroplasma-mediated defense against parasitoid wasps occurs in both male-killing and non-male-killing strains of Spiroplasma associated with Drosophila, and reveals another example of a symbiont that likely uses more than one strategy to ensure persistence. The similar wasp growth inhibitory effects exerted by two different Spiroplasma strains on two wasps with distinct host avoidance/suppression strategies and within two divergent Drosophila hosts suggest that the defensive mechanism is quite general, and probably not associated with enhanced cellular immunity of the host. Furthermore, discovery of symbiont-mediated protection against wasps in a model organism offers a tractable system in which to further explore the defensive mechanism. Finally, the additive positive effect of Spiroplasma and Wolbachia on fly survival against attack by one parasitoid (Lh) constitutes a mechanism by which two, otherwise antagonistic maternally transmitted symbionts, may behave as mutualists.
Data archiving
Data generated from this study have been submitted to Dryad database. Dryad Digital Repository: doi: 10.5061/dryad.47574.
Accession codes
References
Bian GW, Xu Y, Lu P, Xie Y, Xi ZY . (2010). The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathogen 6: e1000833.
Brownlie JC, Johnson KN . (2009). Symbiont-mediated protection in insect hosts. Trends Microbiol 17: 348–354.
Douglas AE . (1998). Nutritional interactions in insect–microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu Rev Entomol 43: 17–37.
Dyer KA, Jaenike J . (2004). Evolutionarily stable infection by a male-killing endosymbiont in Drosophila innubila: molecular evidence from the host and parasite genomes. Genetics 168: 1443–1455.
Engelstadter J, Hurst GDD . (2009). The ecology and evolution of microbes that manipulate host reproduction. Annu Rev Ecol Evol Syst 40: 127–149.
Ferrari J, Vavre F . (2011). Bacterial symbionts in insects or the story of communities affecting communities. Philos Trans R Soc Ser B 366: 1389–1400.
Fleury F, Gibert P, Ris N, Allemand R . (2009). Ecology and life history evolution of frugivorous Drosophila parasitoids. Adv Parasitol 70: 3–44.
Frentiu FD, Robinson J, Young PR, McGraw EA, O'Neill SL . (2010). Wolbachia-mediated resistance to dengue virus infection and death at the cellular level. PLoS One 5: e13398.
Fytrou A, Schofield PG, Kraaijeveld AR, Hubbard SF . (2006). Wolbachia infection suppresses both host defence and parasitoid counter-defence. Proc R Soc Ser B 273: 791–796.
Glaser RL, Meola MA . (2010). The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile Virus infection. PLoS One 5: e11977.
Goto S, Anbutsu H, Fukatsu T . (2006). Asymmetrical interactions between Wolbachia and Spiroplasma endosymbionts coexisting in the same insect host. Appl Environ Microbiol 72: 4805–4810.
Gueguen G, Rajwani R, Paddibhatla I, Morales J, Govind S . (2011). VLPs of Leptopilina boulardi share biogenesis and overall stellate morphology with VLPs of the heterotoma clade. Virus Res 160: 159–165.
Haselkorn TS, Markow TA, Moran NA . (2009). Multiple introductions of the Spiroplasma bacterial endosymbiont into Drosophila. Mol Ecol 18: 1294–1305.
Hedges LM, Brownlie JC, O'Neill SL, Johnson KN . (2008). Wolbachia and virus protection in insects. Science 322: 702.
Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH . (2008). How many species are infected with Wolbachia?—a statistical analysis of current data. FEMS Microbiol Lett 281: 215–220.
Himler AG, Adachi-Hagimori T, Bergen JE, Kozuch A, Kelly SE, Tabashnik BE et al. (2011). Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science 332: 254–256.
Hurst GDD, Hutchence KJ . (2010). Host defence: getting by with a little help from our friends. Curr Biol 20: R806–R808.
Hurst LD . (1991). The incidences and evolution of cytoplasmic male killers. Proc R Soc Ser B 244: 91–99.
Iturbe-Ormaetxe I, Walker T, Neill SLO . (2011). Wolbachia and the biological control of mosquito-borne disease. EMBO Rep 12: 508–518.
Jaenike J . (2012). Population genetics of beneficial heritable symbionts. Trends Ecol Evol 27: 226–232.
Jaenike J, Stahlhut JK, Boelio LM, Unckless RL . (2010a). Association between Wolbachia and Spiroplasma within Drosophila neotestacea: an emerging symbiotic mutualism? Mol Ecol 19: 414–425.
Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ . (2010b). Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science 329: 212–215.
Kacsoh BZ, Schlenke TA . (2012). High hemocyte load is associated with increased resistance against parasitoids in Drosophila suzukii, a relative of D. melanogaster. PLoS One 7: e34721.
Kraaijeveld AR, Barker CL, Godfray HCJ . (2008). Stage-specific sex differences in Drosophila immunity to parasites and pathogens. Evol Ecol 22: 217–228.
Lee MJ, Kalamarz ME, Paddibhatla I, Small C, Rajwani R, Govind S . (2009). Virulence factors and strategies of Leptopilina spp.: selective responses in Drosophila hosts. Adv Parasitol 70: 123–145.
Lemaitre B, Hoffmann J . (2007). The host defense of Drosophila melanogaster. Annu Rev Immunol 25: 697–743.
Longdon B, Fabian D, Hurst G, Jiggins F . (2012). Male-killing Wolbachia do not protect Drosophila bifasciata against viral infection. BMC Microbiol 12: S8.
Lukasik P, van Asch M, Guo H, Ferrari J, Charles JGH, van der Putten W . (2012). Unrelated facultative endosymbionts protect aphids against a fungal pathogen. Ecol Lett 16: 214–218.
Martinez J, Duplouy A, Woolfit M, Vavre F, O’Neill SL, Varaldi J . (2012). Influence of the virus LbFV and of Wolbachia in a host–parasitoid interaction. PLoS One 7: e35081.
Martins AB, Ventura I, Klaczko L . (2010). Spiroplasma infection in Drosophila melanogaster: what is the advantage of killing males? J Invertebr Pathol 105: 145–150.
Mateos M, Castrezana SJ, Nankivell BJ, Estes AM, Markow TA, Moran NA . (2006). Heritable endosymbionts of Drosophila. Genetics 174: 363–376.
Montenegro H, Petherwick A, Hurst G, Klaczko L . (2006). Fitness effects of Wolbachia and Spiroplasma in Drosophila melanogaster. Genetica 127: 207–215.
Montenegro H, Solferini VN, Klaczko LB, Hurst GDD . (2005). Male-killing Spiroplasma naturally infecting Drosophila melanogaster. Insect Mol Biol 14: 281–288.
Montenegro H, Souza WN, Leite DDS, Klaczko LB . (2000). Male-killing selfish cytoplasmic element causes sex-ratio distortion in Drosophila melanogaster. Heredity 85: 465–470.
Morales J, Chiu H, Oo T, Plaza R, Hoskins S, Govind S . (2005). Biogenesis, structure, and immune-suppressive effects of virus-like particles of a Drosophila parasitoid, Leptopilina victoriae. J Insect Physiol 51: 181–195.
Moran NA, McCutcheon JP, Nakabachi A . (2008). Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet 42: 165–190.
Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu GJ, Pyke AT, Hedges LM et al. (2009). A Wolbachia symbiont in Aedes aegypti limits infection with Dengue, Chikungunya, and Plasmodium. Cell 139: 1268–1278.
Nunes MD, Nolte V, Schlotterer C . (2008). Nonrandom Wolbachia infection status of Drosophila melanogaster strains with different mtDNA haplotypes. Mol Biol Evol 25: 2493–2498.
O’Neill SL, Hoffmann AA, Werren JH . (1997) Influential Passengers: Inherited Microorganisms and Arthropod Reproduction. Oxford University Press: New York, NY, USA.
Oliver K, Russell J, Moran N, Hunter M . (2003). Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc Natl Acad Sci USA 100: 1803–1807.
Oliver KM, Moran NA, Hunter MS . (2005). Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proc Natl Acad Sci USA 102: 12795–12800.
Oliver KM, Noge K, Huang EM, Campos JM, Becerra JX, Hunter MS . (2012). Parasitic wasp responses to symbiont-based defense in aphids. BMC Biol 10: 11.
Osborne SE, Leong YS, O'Neill SL, Johnson KN . (2009). Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans. PLoS Pathogen 5: e1000656.
Ota T, Kawabe M, Oishi K, Poulson DF . (1979). Non-male-killing spiroplasmas in Drosophila hydei. J Hered 70: 211–213.
Patot S, Lepetit D, Charif D, Varaldi J, Fleury F . (2009). Molecular detection, penetrance, and transmission of an inherited virus responsible for behavioral manipulation of an insect parasitoid. Appl Environ Microbiol 75: 703–710.
Richardson MF, Weinert LA, Welch JJ, Linheiro RS, Magwire MM, Jiggins FM et al. (2012). Population genomics of the Wolbachia endosymbiont in Drosophila melanogaster. PLoS Genet 8: e1003129.
Riegler M, Sidhu M, Miller WJ, O’Neill SL . (2005). Evidence for a global Wolbachia replacement in Drosophila melanogaster. Curr Biol 15: 1428–1433.
Russo J, Dupas S, Frey F, Carton Y, Brehelin M . (1996). Insect immunity: early events in the encapsulation process of parasitoid (Leptopilina boulardi) eggs in resistant and susceptible strains of Drosophila. Parasitology 112: 135–142.
Scarborough CL, Ferrari J, Godfray HCJ . (2005). Aphid protected from pathogen by endosymbiont. Science 310: 1781–1781.
Schlenke TA, Morales J, Govind S, Clark AG . (2007). Contrasting infection strategies in generalist and specialist wasp parasitoids of Drosophila melanogaster. PLoS Pathogen 3: 1486–1501.
Silva N, Guenther L, Xie J, Mateos M . (2012). Infection densities of three Spiroplasma strains in the host Drosophila melanogaster. Symbiosis 57: 83–93.
Tamura K, Subramanian S, Kumar S . (2004). Temporal patterns of fruit fly (Drosophila) evolution revealed by mutation clocks. Mol Biol Evol 21: 36–44.
Teixeira L, Ferreira A, Ashburner M . (2008). The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6: 2753–2763.
Thomas P, Kenny N, Eyles D, Moreira LA, O'Neill SL, Asgari S . (2011). Infection with the wMel and wMelPop strains of Wolbachia leads to higher levels of melanization in the hemolymph of Drosophila melanogaster, Drosophila simulans and Aedes aegypti. Dev Comp Immunol 35: 360–365.
Unckless RL, Jaenike J . (2012). Maintenance of a male-killing Wolbachia in Drosophila innubila by male-killing dependent and male-killing independent mechanisms. Evolution 66: 678–689.
Varaldi J, Fouillet P, Ravallec M, Lopez-Ferber M, Bouletreau M, Fleury F . (2003). Infectious behavior in a parasitoid. Science 302: 1930–1930.
Varaldi J, Ravallec M, Labrosse C, Lopez-Ferber M, Bouletreau M, Fleury F . (2006). Artifical transfer and morphological description of virus particles associated with superparasitism behaviour in a parasitoid wasp. J Insect Physiol 52: 1202–1212.
Ventura IM, Martins AB, Lyra ML, Andrade CA, Carvalho KA, Klaczko LB . (2012). Spiroplasma in Drosophila melanogaster populations: prevalence, male-killing, molecular identification, and no association with Wolbachia. Microb Ecol 64: 794–801.
Vorburger C, Sandrock C, Gouskov A, Castañeda LE, Ferrari J . (2009). Genotypic variation and the role of defensive endosymbionts in an all-parthenogenetic host–parasitoid interaction. Evolution 63: 1439–1450.
Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ et al. (2011). The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476: 450–U101.
Werren JH, Baldo L, Clark ME . (2008). Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6: 741–751.
White J, Giorgini M, Strand M, Pennacchio F . (2013). Arthropod endosymbiosis and evolution. In: Minelli A, Boxshall G, Fusco G (eds.) Arthropod Biology and Evolution. Springer: Berlin, Heidelberg, Germany. pp 441–477.
Williams MJ, Wiklund ML, Wikman S, Hultmark D . (2006). Rac1 signalling in the Drosophila larval cellular immune response. J Cell Sci 119: 2015–2024.
Xie J, Tiner B, Vilchez I, Mateos M . (2011). Effect of the Drosophila endosymbiont Spiroplasma on parasitoid wasp development and on the reproductive fitness of wasp-attacked fly survivors. Evol Ecol 25: 1065–1079.
Xie J, Vilchez I, Mateos M . (2010). Spiroplasma bacteria enhance survival of Drosophila hydei attacked by the parasitic wasp Leptopilina heterotoma. PLoS One 5: e12149.
Zele F, Nicot A, Duron O, Rivero A . (2012). Infection with Wolbachia protects mosquitoes against Plasmodium-induced mortality in a natural system. J Evol Biol 25: 1243–1252.
Zug R, Hammerstein P . (2012). Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS One 7: e38544.
Acknowledgements
Greg Hurst provided D. melanogaster strains (MSRO-infected Red 42 and Canton-S). Todd Schlenke provided the wasps L. heterotoma (Lh14) and L. boulardi (Lb17). Humberto Martinez collected wild D. melanogaster. Adam Jones, Robert Wharton, Luis Hurtado, Nadisha Silva, Humberto Martinez, Lauryn Winter, Caitlyn Winter and four anonymous reviewers provided helpful suggestions. This study was conducted in partial fulfillment of PhD requirements (JX) at Texas A&M University. Partial funding was provided by NSF Grant DEB 0743782 to Mariana Mateos and Luis A Hurtado. The Chinese Scholarship Council and Texas A&M University provided funding for JX. This is publication no. 233 of the Texas A&M University Center for Biosystematics and Biodiversity.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Heredity website
Supplementary information
Rights and permissions
About this article
Cite this article
Xie, J., Butler, S., Sanchez, G. et al. Male killing Spiroplasma protects Drosophila melanogaster against two parasitoid wasps. Heredity 112, 399–408 (2014). https://doi.org/10.1038/hdy.2013.118
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hdy.2013.118