Abstract
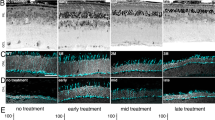
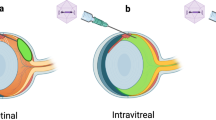
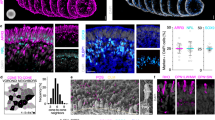
The retinal pigment epithelium (RPE) interacts closely with photoreceptors to maintain visual function. In degenerative diseases such as Stargardt disease and age-related macular degeneration, the leading cause of blindness in the developed world, RPE cell loss is followed by photoreceptor cell death. RPE cells can proliferate under certain conditions, suggesting an intrinsic regenerative potential, but so far this has not been utilised therapeutically. Here, we used E2F2 to induce RPE cell replication and thereby regeneration. In both young and old (2 and 18 month) wildtype mice, subretinal injection of non-integrating lentiviral vector expressing E2F2 resulted in 47% of examined RPE cells becoming BrdU positive. E2F2 induced an increase in RPE cell density of 17% compared with control vector-treated and 14% compared with untreated eyes. We also tested this approach in an inducible transgenic mouse model of RPE loss, generated through activation of diphtheria toxin-A gene. E2F2 expression resulted in a 10-fold increase in BrdU uptake and a 34% increase in central RPE cell density. Although in mice this localised rescue is insufficiently large to be demonstrable by electroretinography, a measure of massed retinal function, these results provide proof-of-concept for a strategy to induce in situ regeneration of RPE for the treatment of RPE degeneration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gehrs KM, Anderson DH, Johnson LV, Hageman GS . Age-related macular degeneration–emerging pathogenetic and therapeutic concepts. Ann Med 2006; 38: 450–471.
Klein R, Klein BE, Jensen SC, Meuer SM . The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology 1997; 104: 7–21.
Klein R, Klein BE, Linton KL . Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology 1992; 99: 933–943.
Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004; 122: 564–572.
Strauss O . The retinal pigment epithelium in visual function. Physiol Rev 2005; 85: 845–881.
Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP . Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol 2003; 48: 257–293.
Dorey CK, Wu G, Ebenstein D, Garsd A, Weiter JJ . Cell loss in the aging retina. Relationship to lipofuscin accumulation and macular degeneration. Invest Ophthalmol Vis Sci 1989; 30: 1691–1699.
Marshall J . The ageing retina: physiology or pathology. Eye (Lond) 1987; 1 (Pt 2): 282–295.
Kaldarar-Pedotti S . [Mitotic activity of the pigment epithelium during embryonic and postembryonic development]. Adv Ophthalmol 1979; 39: 37–58.
Del Priore LV, Kuo YH, Tezel TH . Age-related changes in human RPE cell density and apoptosis proportion in situ. Invest Ophthalmol Vis Sci 2002; 43: 3312–3318.
Longbottom R, Fruttiger M, Douglas RH, Martinez-Barbera JP, Greenwood J, Moss SE . Genetic ablation of retinal pigment epithelial cells reveals the adaptive response of the epithelium and impact on photoreceptors. Proc Natl Acad Sci USA 2009; 106: 18728–18733.
Machemer R, Steinhorst UH . Retinal separation, retinotomy, and macular relocation: II. A surgical approach for age-related macular degeneration? Graefes Arch Clin Exp Ophthalmol 1993; 231: 635–641.
Eandi CM, Giansanti F, Virgili G . Macular translocation for neovascular age-related macular degeneration. Cochrane Database Syst Rev 2008; 8: CD006928.
MacLaren RE, Bird AC, Sathia PJ, Aylward GW . Long-term results of submacular surgery combined with macular translocation of the retinal pigment epithelium in neovascular age-related macular degeneration. Ophthalmology 2005; 112: 2081–2087.
da CL, Chen FK, Ahmado A, Greenwood J, Coffey P . RPE transplantation and its role in retinal disease. Prog Retin Eye Res 2007; 26: 598–635.
Binder S, Stanzel BV, Krebs I, Glittenberg C . Transplantation of the RPE in AMD. Prog Retin Eye Res 2007; 26: 516–554.
van Romunde SH, Polito A, Bertazzi L, Guerriero M, Pertile G . Long-term results of full macular translocation for choroidal neovascularization in age-related macular degeneration. Ophthalmology 2015; 122: 1366–1374.
Aisenbrey S, Lafaut BA, Szurman P, Hilgers RD, Esser P, Walter P et al. Iris pigment epithelial translocation in the treatment of exudative macular degeneration: a 3-year follow-up. Arch Ophthalmol 2006; 124: 183–188.
Joussen AM, Joeres S, Fawzy N, Heussen FM, Llacer H, van Meurs JC et al. Autologous translocation of the choroid and retinal pigment epithelium in patients with geographic atrophy. Ophthalmology 2007; 114: 551–560.
MacLaren RE, Uppal GS, Balaggan KS, Tufail A, Munro PM, Milliken AB et al. Autologous transplantation of the retinal pigment epithelium and choroid in the treatment of neovascular age-related macular degeneration. Ophthalmology 2007; 114: 561–570.
van Meurs JC, ter AE, Croxen R, Hofland L, van Hagen PM . Comparison of the growth potential of retinal pigment epithelial cells obtained during vitrectomy in patients with age-related macular degeneration or complex retinal detachment. Graefes Arch Clin Exp Ophthalmol 2004; 242: 442–443.
Gouras P, Flood MT, Kjedbye H, Bilek MK, Eggers H . Transplantation of cultured human retinal epithelium to Bruch's membrane of the owl monkey's eye. Curr Eye Res 1985; 4: 253–265.
Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 2015; 385: 509–516.
Cyranoski D . Stem cells cruise to clinic. Nature 2013; 494: 413.
Song P, Inagaki Y, Sugawara Y, Kokudo N . Perspectives on human clinical trials of therapies using iPS cells in Japan: reaching the forefront of stem-cell therapies. Biosci Trends 2013; 7: 157–158.
Dyson N . The regulation of E2F by pRB-family proteins. Genes Dev 1998; 12: 2245–2262.
Nevins JR . The Rb/E2F pathway and cancer. Hum Mol Genet 2001; 10: 699–703.
Wu L, Timmers C, Maiti B, Saavedra HI, Sang L, Chong GT et al. The E2F1-3 transcription factors are essential for cellular proliferation. Nature 2001; 414: 457–462.
DeGregori J, Leone G, Miron A, Jakoi L, Nevins JR . Distinct roles for E2F proteins in cell growth control and apoptosis. Proc Natl Acad Sci USA 1997; 94: 7245–7250.
Albert DM, Tso MO, Rabson AS . In vitro growth of pure cultures of retinal pigment epithelium. Arch Ophthalmol 1972; 88: 63–69.
Machemer R, Laqua H . Pigment epithelium proliferation in retinal detachment (massive periretinal proliferation). Am J Ophthalmol 1975; 80: 1–23.
Machemer R, van HD, Aaberg TM . Pigment epithelial proliferation in human retinal detachment with massive periretinal proliferation. Am J Ophthalmol 1978; 85: 181–191.
Anderson DH, Stern WH, Fisher SK, Erickson PA, Borgula GA . The onset of pigment epithelial proliferation after retinal detachment. Invest Ophthalmol Vis Sci 1981; 21: 10–16.
Ach T, Huisingh C, McGwin G Jr, Messinger JD, Zhang T, Bentley MJ et al. Quantitative autofluorescence and cell density maps of the human retinal pigment epithelium. Invest Ophthalmol Vis Sci 2014; 55: 4832–4841.
Al-Hussaini H, Kam JH, Vugler A, Semo M, Jeffery G . Mature retinal pigment epithelium cells are retained in the cell cycle and proliferate in vivo. Mol Vis 2008; 14: 1784–1791.
Georgiadis A, Tschernutter M, Bainbridge JW, Balaggan KS, Mowat F, West EL et al. The tight junction associated signalling proteins ZO-1 and ZONAB regulate retinal pigment epithelium homeostasis in mice. PLoS One 2010; 5: e15730.
Tamiya S, Liu L, Kaplan HJ . Epithelial-mesenchymal transition and proliferation of retinal pigment epithelial cells initiated upon loss of cell-cell contact. Invest Ophthalmol Vis Sci 2010; 51: 2755–2763.
Bracken AP, Ciro M, Cocito A, Helin K . E2F target genes: unraveling the biology. Trends Biochem Sci 2004; 29: 409–417.
Jirawatnotai S, Sharma S, Michowski W, Suktitipat B, Geng Y, Quackenbush J et al. The cyclin D1-CDK4 oncogenic interactome enables identification of potential novel oncogenes and clinical prognosis. Cell Cycle 2014; 13: 2889–2900.
Galla M, Schambach A, Towers GJ, Baum C . Cellular restriction of retrovirus particle-mediated mRNA transfer. J Virol 2008; 82: 3069–3077.
Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C et al. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of imunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther 2002; 13: 803–813.
Yanez-Munoz RJ, Balaggan KS, MacNeil A, Howe SJ, Schmidt M, Smith AJ et al. Effective gene therapy with nonintegrating lentiviral vectors. Nat Med 2006; 12: 348–353.
Bainbridge JWB, Stephens C, Parsley K, Demaison C, Halfyard A, Thrasher AJ et al. In vivo gene transfer to the mouse eye using an HIV-based lentiviral vector; efficient long-term transduction of corneal endothelium and retinal pigment epithelium. Gene Ther 2001; 8: 1665–1668.
Ali RR, Reichel MB, Thrasher AJ, Levinsky RJ, Kinnon C, Kanuga N et al. Gene transfer into the mouse retina mediated by an adeno-associated viral vector. Hum Mol Genet 1996; 5: 591–594.
Acknowledgements
This work was supported by the National Institute for Health Research Biomedical Research Centre at Moorfields Eye Hospital and UCL, and also by Moorfields Eye Charity and RP Fighting Blindness. The authors gratefully acknowledge Anselm Kampik, Augenzentrum im Brienner Hof, Munich, Germany, for helpful discussions. The authors thank Nancy Joyce, Schepens Eye Research Institute, Harvard Medical School, Boston, USA, for providing the human E2F2 cDNA plasmid.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
UFOL is employee of F Hoffmann-La Roche Ltd. The remaning authors declare no conflict interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Kampik, D., Basche, M., Luhmann, U. et al. In situ regeneration of retinal pigment epithelium by gene transfer of E2F2: a potential strategy for treatment of macular degenerations. Gene Ther 24, 810–818 (2017). https://doi.org/10.1038/gt.2017.89
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2017.89
This article is cited by
-
A CRISPR-Cas9-mediated F0 screen to identify pro-regenerative genes in the zebrafish retinal pigment epithelium
Scientific Reports (2023)
-
A 2020 vision of ocular gene therapy
Gene Therapy (2021)
-
Macular hyperpigmentary changes in ABCA4-Stargardt disease
International Journal of Retina and Vitreous (2019)