Abstract
Retroviral vectors including lentiviral vectors are commonly used tools to stably express transgenes or RNA molecules in mammalian cells. Their utilities are roughly divided into two categories, stable overexpression of transgenes and RNA molecules, which requires maximal transduction efficiency, or functional selection with retrovirus (RV)-based libraries, which takes advantage of retroviral superinfection resistance. However, the dynamic features of RV-mediated transduction are not well characterized. Here, we engineered two murine stem cell virus-based retroviral vectors expressing dual fluorescence proteins and antibiotic markers, and analyzed virion production efficiency and virion stability, dynamic infectivity and superinfection resistance in different cell types, and strategies to improve transduction efficiency. We found that the highest virion production occurred between 60 and 72 h after transfection. The stability of the collected virion supernatant decreased by >60% after 3 days in storage. We found that RV infectivity varied drastically in the tested human cancer lines, while low transduction efficiency was partially overcome with increased virus titer, prolonged infection duration and/or repeated infections. Furthermore, we demonstrated that RV receptors PIT1 and PIT2 were lowly expressed in the analyzed cells, and that PIT1 and/or PIT2 overexpression significantly improved transduction efficiency in certain cell lines. Thus, our findings provide resourceful information for the optimal conditions of retroviral-mediated gene delivery.
Similar content being viewed by others
Introduction
Retroviruses (RVs) contain a non-segmented RNA genome, and their hallmark is a replicative strategy, which includes reverse transcription of the virion RNA into linear double-stranded DNA (also known as provirus) and the subsequent integration of this DNA into the host genome.1, 2, 3, 4, 5 The provirus is transcribed into mRNAs that encode the viral proteins, which subsequently package the full‐length genomic mRNA into virions to complete the virus life cycle. RVs comprise a large and diverse family of enveloped RNA viruses defined by common taxonomic denominators that include structure, composition and replicative properties.1, 3, 4, 5, 6 Traditionally, RVs have been broadly divided into two categories, simple RVs (for example, Moloney murine leukemia virus and murine stem cell virus) and complex RVs (for example, lentiviruses including human T-cell leukemia virus), which are distinguishable by their genome organization.2, 3, 4, 5, 6 All RVs contain three essential coding domains with information for virion proteins: gag, which encodes internal virion proteins that form the matrix, the capsid and the nucleoprotein structures; pol, which encodes reverse transcriptase and integrase; and env, which encodes the surface and transmembrane components of the viral envelope proteins.6, 7, 8 In addition to these proteins, the lentiviruses and spumaviruses require accessory proteins that modulate various viral and cellular functions involved in virus gene expression and interaction with the host animal.7, 8 The study of RV biology has impacted broad areas of molecular biology and medicine, including the study of cellular growth control, and carcinogenesis.1, 3, 4, 5, 9
For the past decades, retroviral vectors have become one of the most commonly used tools to transfer and integrate specific DNA sequences into the host genome, and are often designed to be replication‐defective to avoid further spread after the initial transfer event.10, 11 Retroviral vectors derived from different RV groups are mostly distinguished by their abilities to transduce nondividing cells, with the simple/oncogenic RVs usually being unable to transduce nondividing cells, while lentiviral vectors being able to transduce nondividing cells.10, 11 The widely available retroviral vectors and packaging cell lines or packaging plasmids have placed the retroviral technology within the reach of any laboratory with standard cell culture and recombinant DNA expertise.
The in vitro applications of retroviral vectors can be roughly divided into two categories: stable overexpression of transgenes or RNA molecules; and functional selection assays using RV-based expression libraries. To achieve high levels of stable overexpression of transgenes or RNA species, such as short interfering RNAs, small hairpin RNAs, short guide RNAs, long noncoding RNAs or microRNAs, it is critical to maximize retroviral transduction efficiency of target cells. Conversely, for retroviral vector-based library-screening studies (such as short interfering RNA, small hairpin RNA or short guide RNA of CRISPR/Cas9 libraries) it is essential to maintain stable single-virus entry into each of the target cells in order to simplify the validation of genotype–phenotype correlations. Retroviral vectors are particularly suited for such library-based functional selection studies due to the well-recognized superinfection resistance of RV infection.1, 2, 3, 4, 5 The mono-viral infection phenomenon of RVs has become even more important as RV-based library selections have been used for functional screening. However, the dynamic features of RV-mediated transgene and RNA expression have not been thoroughly characterized.
In this study, using the murine stem cell virus-based retroviral vectors we analyze the virion production efficiency and stability of RV virions, the dynamic changes of the infectivity and superinfection resistance in various cell types, and effective strategies to improve the infectivity and transduction efficiency of RVs. Thus, our findings provide a practical and valuable guide for the optimal usage of retroviral vectors for in vitro cell culture studies and in vivo animal experiments.
Results
The RV packaging timeline indicates the most effective viral production occurs at 60–72 h after transfection
Even though retroviral vectors are widely used for stable gene delivery, many aspects involved in the production and infectivity of RV have not been thoroughly analyzed. We constructed two RV transfer vectors that express a distinct set of fluorescent proteins (enhanced green fluorescent protein or monomeric red fluorescent protein) and antibiotic selection markers (neo/G418 or blasticidin S resistance marker; BSD), RV-GN and RV-RB (Figure 1A). We first determined the optimal timeline for collecting the packaged RV particles by collecting the RV supernatants in 12 h intervals starting at 24 h after transfection (Figure 1Ba). The same percentage of the RV supernatants collected at the five time points was used to infect human melanoma lines A375 and MDA-MB-435 cells. We found that under the same infection conditions for both cell lines the 72 h RV supernatant (72hSup) yielded the highest numbers of surviving clones, followed by 60hSup, while 24hSup produced lowest numbers of surviving clones, qualitatively and quantitatively (Figure 1Bb vs c). The pooled RV supernatants of all five time points yielded surviving clones similar to that of 60hSup (Figures 1Bb and c). The quantitative data also indicated that the infected A375 cells yielded more surviving clones than that of MDA-MB-435 cells for four of the five tested RV preparations, suggesting that A375 cells may be more susceptible to RV infection.
The titers of packaged RVs peak at 60–72 h in the transfected packaging cells. (A) Schematic representation of the two retroviral vectors RV-RB (co-expressing BSD and mRFP or monomeric red fluorescent protein) and RV-GN (co-expressing neo/G418 resistance marker and eGFP or enhanced green fluorescent protein). BSD, blasticidin S resistance marker; eGFP, enhanced green fluorescent protein, enhanced green fluorescent protein; hEFH, a hybrid promoter containing human elongation factor 1α promoter and human HIV enhancer; mRFP, monomeric red fluorescent protein; neo/G418, neo/G418 resistance marker. (B) Determination of the optimal time points for RV packaging. (a) A flowchart depicting the time point-dependent collections of RV supernatant (for example, 24 hSup to 72hSup) after transfection (Txn) of retroviral transfer vectors and packaging vector pCL-Ampho. (b) A375 and MDA-MB-435 cells were infected with RV-GN or RV-RB supernatant prepared from the indicated time points for 24 h and selected in G418 or BSD for 5 days. ‘Mix’ indicated the pooled virus supernatants from all five time points. Cells were fixed and stained with crystal violet. Each assay condition was done in triplicate and representative results are shown. (c) Quantitative measurement of crystal violet-stained cells in b.
RV superinfection occurs more often with longer infection duration and/or higher RV titers
It is well established that RV infection renders the target cells temporally refractory to superinfection although this phenomenon has not been thoroughly examined. Using the low-titer (24hSup) and high-titer (60hSup and 72hSup) samples for both RV-GN and RV-RB, we analyzed the nature of superinfection under different lengths of infection and found that the double-resistance clones were rarely observed in low titer (24hSup) even when the cells were infected for up to 24 h (Figures 2Aa and a′), indicating that almost all cells were infected by either RV-GN or RV-RB, but not both viruses. However, when A375 cells were infected with higher-titer RV preparations (60hSup and 72hSup), double-resistance clones were readily obtained even when the cells were infected for 4 h, although more double-resistance clones were observed when the infection durations increased to 8, 12 or 24 h (Figures 2Ab and c, and b′ and c′).
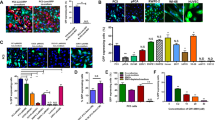
Longer infection duration increases superinfection of RVs. (A) RV supernatants collected at 24, 60 and 72 h were used to infect subconfluent A375 cells for the indicated duration (for example, 4–24 h). At the end of infection, medium was changed. The infected cells were selected with antibiotics at 24 h after infection. Cells were stained with crystal violet at 5 days after selection (a–c). Each assay condition was done in triplicate and representative results are shown. The stained cells were dissolved and quantitatively measured (a′–c′). ‘B+G’, blasticidin S resistance marker (BSD) and G418 double-selection. (B) Fluorescence detection of the superinfected cells. Subconfluent A375 cells were infected with the equal titers of RV-GN and RV-RB virus supernatant for 2, 4 and 8 h. Green fluorescent protein (GFP) and red fluorescent protein (RFP) expression was detected at 36 h after infection. The merged GFP and RFP expression is shown. Representative superinfected cells were indicated by arrows. (C) Superinfected cells maintain long-term co-expression of GFP and RFP markers. The RV-GN- and RV-RB-infected A375 cells were selected in BSD and G418 for 7 days and examined for GFP and RFP expression. Both signals were merged and representative superinfected cells are indicated by arrows. Representative images are shown.
Superinfection can be further observed by examining fluorescent protein expression in the infected cells even at the early stage of infection. Using high-titer RV-GN and/or RV-RB supernatants (60hSup) to infect A375 cells, we found that dual fluorescence-positive cells were rarely observed in the cells only infected for 2 h, while significantly increased in 4 h- and 8 h-infection groups (Figure 2B), indicating that superinfection may occur more often in longer infection durations. Furthermore, the dual-resistance clones obtained under different infection durations exhibited strong dual fluorescence (Figure 2C). Taken together, these results suggest that RV superinfection may occur more frequently with longer duration of infection and/or high-titer counts.
Packaged RVs have a limited stability in storage
It is a common practice to collect and pool the RV supernatants from different time points after transfection and then to infect target cells after the last collection. However, the stability of the collected RV supernatants in storage is not known. Using the same starting titers of RV-GN and RV-RB supernatants, which were kept at 4 °C for 0–8 days, we infected A375 cells for different durations and found that surviving single-resistance cells remained rather steady for the RV samples kept at 4 °C for 2 days, while double-resistance clones increased with the infection durations(Figures 3Aa–c). However, RV infectivity decreased drastically for the RV supernatants kept at 4 °C for 3 days and was almost lost completely after 7 days in storage (Figures 3Ad–i).
Packaged RV supernatants have limited stability in storage. (A) Virus titers decrease remarkably after 3-day storage at 4 °C. Equal titers of RV-GN and RV-RB virus supernatants were collected, stored at 4 °C for indicated days and were used to infect A375 cells for the indicated duration. At 24 h after infection, cells were selected with blasticidin S resistance marker (BSD) and/or G418 (B+G, BSD and G418 double-selection), and subjected to crystal violet staining at 5 days after selection (a–i). (B) Quantitative determination of virus supernatant stability at 4 °C. The crystal violet-stained cells infected with the virus supernatants kept at 4 °C for various days for 4 (a), 12 (b) and 24 h (c) were quantitatively measured.
Quantitatively, in the 4 h-infection group the numbers of surviving drug-resistant cells remained unchanged in day 0 (freshly prepared and used immediately), day 1 and day 2 storage samples while the average infectivity significantly decreased to 40, 31, 31, 16, 12 and 8 of that of the freshly prepared RV supernatants in the day 3, 4, 5, 6, 7 and 8 storage samples, respectively (Figure 3Ba). In the 12 h-infection group, the infectivity did not change significantly for the RV preparations stored for up to 3 days, but the average infectivity significantly decreased to 43, 36, 24, 19 and 15 of that of the freshly prepared RV supernatants in the day 4, 5, 6, 7 and 8 storage samples, respectively (Figure 3Bb). Finally, in the 24 h-infection group the average infectivity was significantly decreased in after 5 days in storage (38, 26, 15 and 13% of that for the fresh RV supernatant; Figure 3Bc). The average titers of the RV supernatants decreased significantly after 3 days in storage. Interestingly, longer infection time can partially compensate for the decreased infectivity. For example, the 4 h infection with the 3-day storage RV samples yielded only 40% of the infectivity of the fresh RV supernatants, whereas12 and 24 h infection durations exhibited similar infectivity to that of the freshly prepared RV supernatants. Nonetheless, the infectivity of the RV supernatants decreased significantly 4 days after storage regardless of infection durations, as the infectivity dropped to 31%, 43% and 64% of that for the freshly prepared RV supernatants for 4, 12 and 24 h infection durations, respectively. Thus, the packaged RV-containing supernatants should be used to infect target cells as freshly as possible.
RV infectivity varies drastically among cell lines and low infection efficiency can be partially overcome by increasing virus titer or prolonging infection duration
As RV virions are usually transiently packaged and directly used to infect target cells, differences in infection efficiency are commonly attributed to virus titer variations. Thus, the RV infectivity among different cell lines/types is rarely analyzed. Here we analyzed a panel of human cancer lines for their RV infection efficiency. Using the same RV titer, we found that A375 cells exhibited the highest infection efficiency, followed by human osteosarcoma line 143B cells, whereas human osteosarcoma line MG63, human glioblastoma lines Duke263 and U251, and human breast cancer line HCC1954 cells only showed marginal infection (Figure 4A), indicating a wide range of infectivity among these six lines. We also tested RV infectivity in two melanoma lines A375 and MDA-MB-435 lines with different virus titers and varied infection duration. We found while under our assay conditions A375 cells were well infected even with the lowest virus titer (for example, 24hSup) and for the shortest infection duration (for example, 4 h), MDA-MB-435 cells were infected more efficiently with either higher virus titers and/or longer infection duration (Figure 4Ba vs b).
RV exhibits drastically different infection efficiencies among cell lines. (A) RV exhibits significantly different infectivity among cell lines. Equal titer of RV-RB virus was used to infect the six cell lines for 8 h and selected with or without blasticidin S resistance marker (BSD) for 5 days, followed by crystal violet staining. Each assay condition was done in triplicate, and representative results are shown. (B) Difference in RV infection efficiency in A375 and MDA-MB-435 cells. Subconfluent A375 (a) and MDA-MB-435 (b) cells were infected with various RV-RB supernatants for different durations. The infected cells were selected with BSD for 5 days, followed by crystal violet staining. Each assay condition was done in duplicate, and representative results are shown.
We further tested the effect of RV titers on infection efficiency for the cells with low RV infectivity. Using U251 cells we found that the infection efficiency was proportionally related to the RV virus titers as the twofold serial dilutions of the RV supernatants yielded a gradient of decreased infectivity (Figures 5Aa and b). Moreover, while longer infection time (for example, 4 vs 8 h) enhanced infection efficiency (P<0.001), we found that repeated two to four cycles of infections with the same RV supernatants significantly improved the overall infection efficiency in U251 cells (Figures 5Ba and b). These results strongly indicate that using high-titer viruses, prolonging infection time and repeated infections may be practical and effective strategies to improve RV infection efficiency in low-infectivity cells.
Low RV infectivity can be partially overcome by increasing virus titers, prolonged infection duration and/or repeated infections. (A) Subconfluent U251 cells were infected with twofold serially diluted RV-RB supernatant for 8 h. The cells were selected with blasticidin S resistance marker (BSD) for 5 days and stained with crystal violet (a), followed by quantitative measurements (b). Each assay condition was done in triplicate, and representative results are shown. (B) Subconfluent U251 cells were infected with the fixed titer of RV-RB supernatant for 4, 8 or multiple rounds of 4 h infection (2 ×, 3 × and 4 ×). The cells were selected with BSD for 5 days and stained with crystal violet (a), followed by quantitative measurements (b). Each assay condition was done in triplicate, and representative results are shown.
Exogenous expression of RV receptors PIT1 and/or PIT2 improves infectivity in certain cell lines
An alternative strategy to improve RV infectivity is to facilitate RV interaction and internalization with RV cell surface receptors. PIT1 and PIT2 have been reported to act as RV receptors.12, 13 We first analyzed the endogenous expression levels of PIT1 and PIT2 in a panel of six human cancer lines. The quantitative PCR (qPCR) analysis revealed that both PIT1 and PIT2 were expressed at relatively low levels in these lines, whereas PIT1 had a higher expression level than that of PIT2 in each of the analyzed cell lines (Figure 6A). Nonetheless, the PIT1 and/or PIT2 expression levels were not completely correlated with RV infectivity as A375 did not exhibit the highest levels of PIT1 and/or PIT2 expression (Figure 6A).
Exogenous expression of RV receptors PIT1 and/or PIT2 improves infectivity in certain cell lines. (A) Endogenous expression levels of PIT1 and PIT2 in the six cell lines as determined by touchdown qPCR. GAPDH was used as the reference gene. (B) Subconfluent MG63 (a), HCC1954 (b) and U251 (c) cells were infected with low, medium and high titers of AdPIT1 and/or AdPIT2, or AdGFP adenoviruses for 24 h, and then infected with a fixed titer of RV-RB RV for 4 h. At 24 h after RV-RB infection, the cells were selected with or without blasticidin S resistance marker (BSD) for 5 days and stained with crystal violet, followed by quantitative measurements. **P<0.001 vs that of the AdGFP-infected cells.
To effectively overexpress PIT1 or PIT2 in target cells, we constructed and generated two adenoviruses expressing human PIT1 and PIT2, and AdPIT1 and AdPIT2, respectively, using our AdEasy system.14, 15, 16 We infected the target cell lines with AdPIT1 and/or AdPIT2, or adenovirus expressing green fluorescent protein (AdGFP) control adenoviruses in three representative cell lines MG63, HCC1954 and U251. Adenovirus infection led to a drastic overexpression of exogenous PIT1 or PIT2 in the infected cell lines (data not shown). We found that overexpression of PIT1, PIT2, and PIT1/PIT2 at low, medium and high titers in MG63 cells significantly increased the infectivity of the RV RV-RB by average 5.1-, 5.5- and 6.0-fold, respectively, compared with that of AdGFP-infected MG63 cells (P<0.001; Figure 6Ba). Moreover, we found that exogenous expression of either PIT1 and/or PIT2 had similar effects on RV-RB infectivity. For the HCC1954 cells, low, medium and high titers of AdPIT1, AdPIT2 and AdPIT1/AdPIT2 infection led to average 2.5-, 2.8- and 2.6-fold increases in RV-RB infectivity (P<0.001) although the overall infectivity in HCC1954 cells was significantly lower than that for MG63 cells (Figure 6Bb). However, overexpression of PIT1, PIT2 and PIT1/PIT2 in U251 cells did not improve the infection efficiency of the RV-RB RV (Figure 6Bc). Taken together, these results suggest that overexpression of PIT1 and/or PIT2 may significantly improve RV infection efficiency for a subgroup of cell lines but not for all cell lines.
Discussion
Although retroviral vectors including lentiviral vectors have been widely used as an effective gene transfer tool in cultured cells and/or animal experiments,11, 17 many parameters that may affect the efficiency of the retroviral vector-mediated gene transduction have not been thoroughly examined. Here, by taking advantage of dual fluorescence protein markers and dual antibiotic selection markers, we evaluated murine stem cell virus for its virion packaging efficiency, stability of RV virions and the dynamic infectivity in various cell lines. We found that the highest virion production period occurs between 60 and 72 h after transfection of packaging cells. The collected virion-containing supernatant has relatively limited stability and exhibits a significantly diminished infectivity after 3 days in storage. We also found that superinfection resistance can be overcome by longer infection time and/or higher viral titers. Finally, we demonstrated that overexpression of retroviral receptors PIT1 and/or PIT2 can significantly enhance viral infectivity and gene transfer in certain cell lines. Thus, our findings provide a practical and valuable guide for an optimal usage of retroviral vectors for in vitro cell culture studies and in vivo animal experiments.
High transduction efficiency and overcoming superinfection resistance are beneficial for achieving high levels of transgene expression. In general, RVs enter target cells through the attachment of their surface glycoproteins to specific cell membrane receptors, leading to fusion of virus and cell membranes.3, 4, 5 The glycosylated hydrophilic polypetide, so called surface unit and membrane-spanning protein, so called transmembrane unit of the envelope glycoprotein complex of RVs have an essential role in the virus entry process.3, 4, 5 The interaction of RVs and cell surfaces is considered rather specific as it constitutes the main determinant of viral host range and defining susceptible animal species, as well as the target cells within the host.3, 4, 5 The surface unit protein binds to a specific receptor on the target cell, and the specificity of the surface unit/receptor interaction defines the host range and tissue tropism of a RV as viral particles lacking envelope glycoproteins are noninfectious while cells lacking a receptor are nonpermissive for viral entry.3, 4, 5 Numerous receptors for retroviral entry have been identified and characterized to date, but they appear to be distinct for the different major viral subgroups, and no clear association between their normal cell function and their receptor activity is apparent.18, 19
It has been well established that human cells express distinct but related receptors for the gibbon ape leukemia virus and the amphotropic murine leukemia virus, termed PIT1 and PIT2, respectively.12, 13, 20, 21 We found that the expression levels of PIT1 and/or PIT2 were generally low and varied significantly in the tested human cancer lines while their expression levels were not perfectly correlated with RV infectivity. Nonetheless, we demonstrated that forced expression of PIT1 and/or PIT2 can significantly improve the RV infectivity of certain cell lines. While molecular details about how RVs’ entry into target cells is regulated are not known, our results indicate that RV infection efficiency can be effectively improved by increasing virus titers, prolonging infection durations, repeating infection cycles and/or overexpressing PIT1/PI2 receptors.
Another commonly used strategy to overcome RV tropism and/or to increase viral transduction efficiency is to generate pseudotyped RVs by incorporating the unrelated rhabdovirus vesicular stomatitis virus G protein into the functional Env proteins during virion production/packaging.22, 23 This strategy allows a co-infection of a single cell with multiple copies of the same or different RVs, overcoming the limit of restricted host-cell range and low titer of retroviral vectors in eukaryotic cells, including non-mammalian species such as zebrafish.22 However, the vesicular stomatitis virus G protein-pseudotyped RVs (including lentiviruses) have to be pre-titered if single-copy infection is desired, such as for small hairpin RNA or CRISPR library selections. In these cases, the multiplicities of infection are usually <1.0 (for example, 0.5 viral particle per cell) to ensure mono-infection of the target cells.
One of the most attractive utilities of retroviral vectors is to transduce target cells with a single copy of the transcripts of interest (for example, cDNA libraries, small hairpin RNA libraries or short guide RNA libraries) integrated into the host genome. It has been well established that prior infection of cells with a RV blocks infection of the same cells by a RV that utilizes the same receptor, a process known as superinfection resistance or viral interference.1, 2, 3, 4, 5 This process results from masking or downregulation of receptors following interaction with the glycoprotein of the established virus, which is probably caused by a direct interaction between the Env protein and the receptor within the secretory pathway or on the cell surface that can then lead to degradation of the complex.24 Viral interference may have an important role in vivo by limiting the growth of exogenous or activated endogenous viruses as such resistance is probably a selected feature of endogenous proviruses as many of them express Env gene products but are defective for virion formation.1, 2, 3, 4, 5
Mechanistically, it was shown that in rough endoplasmic reticulum expression of a mutant glycoprotein, which was trapped in the endoplasmic reticulum, prevented superinfection of cells by wild-type virus, and this mutant Env presumably interacted with and blocked the transport of the receptor to the cell surface.24 Such effects are probably important not only for inducing superinfection resistance but also for permitting efficient virion release by blocking or reversing premature Env–receptor interaction.25 While the molecular mechanisms underlying retroviral interference are likely complex and remain to be thoroughly understood, such superinfection resistance is critical for expression library-based functional selection studies. Currently, it is not clearly demonstrated how long a viral interference period would last during a single infection. Our experimental findings reveal that the surperinfection resistance can be comprised if high titers of RVs are used and/or a prolonged infection is carried out. Thus, it is critical to determine the infectivity of target cells, optimal titer of RVs and appropriate length of infection of target cells when retroviral vectors are used to deliver expression libraries for functional selections.
In summary, using dual fluorescence protein markers and dual antibiotic selection markers, we investigated retroviral virion packaging efficiency, stability of RV virions and the dynamic infectivity in various cell lines. We found that the highest virion production period occurs between 60 and 72 h after transfection. The stability and infectivity of the collected virion supernatant decreased significantly after 3 days in storage. Furthermore, we demonstrated that longer infection time and/or higher viral titers can overcome RV superinfection resistance. Finally, we showed that overexpression of retroviral receptors PIT1 and/or PIT2 can significantly enhance viral infectivity in certain cell lines. Thus, these findings provide resourceful information for the optimal usage of retroviral vector-mediated gene delivery.
Materials and methods
Cell culture and chemicals
Phoenix-AMPHO, human melanoma lines A375 and MDA-MB-435, human osteosarcoma lines 143B and MG63, human breast cancer line HCC1954, and human glioblastoma line U251 cells were obtained from American Type Culture Collection (Manassas, VA, USA). Human glioblastoma line Duke263 was generously provided by Dr Darell D Bigner of Duke University. 293pTP cells were derived from HEK-293 cells and express high level of human Ad5 pTP gene, as previously characterized.26 The iMEF cells are mouse mesenchymal stem cells previously described.27, 28 These cells lines were maintained in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum (Gemini Bio-Products, West Sacramento, CA, USA), 100 U ml−1 penicillin and 100 g ml−1 streptomycin at 37 °C in 5% CO2 as described.29, 30 Unless indicated otherwise, all other reagents were purchased from Sigma-Aldrich (St Louis, MO, USA) or Thermo Fisher Scientific (Waltham, MA, USA).
Construction of retroviral vectors RV-GN and RV-RB
The retroviral transfer vectors pRV-GN and pRV-RB were constructed using our homemade murine stem cell virus-based pSEB and pSEN retroviral vectors.31 The pRV-RB vector co-expresses blasticidin S resistance marker and monomeric red fluorescent protein, while the pRV-GN vector co-expresses neo/G418 resistance marker and enhanced green fluorescent protein. Both blasticidin S resistance marker and neo/G418 drug resistance marker are driven by the retroviral 5′-long terminal repeat, whereas the expression of monomeric red fluorescent protein or enhanced green fluorescent protein is driven by a hEFH, a hybrid promoter containing human elongation factor 1α promoter and human HIV enhancer.31, 32 The vectors were constructed by PCR cloning, and all PCR-amplified fragments were verified by DNA sequencing. Detailed information regarding vector constructions is available upon request.
Transfection, RV packaging and infection
RV packaging was carried out by co-transfecting the retroviral transfer vector pRV-GN or pRV-RB and pCL-Ampho packaging plasmid into Phoenix-AMPHO cells. Briefly, subconfluent Phoenix-AMPHO cells seeded in T-25 cell culture flasks were transfected with the plasmid DNA-PEI mixture of 3 μg of RV transfer vector, 3 μg of pCL-Ampho and 45 μg PEI (Polysciences Inc., Warrington, PA, USA) under the serum-free condition for 2–4 h. RV supernatants were collected at 36, 48, 60 and 72 h after transfection. The collected RV supernatants were centrifuged to remove cell debris and kept at 4 °C till use.
For RV infection, cells were freshly seeded at subconfluence and RV supernatants were added to the culture medium in the presence of polybrene (at 4 μg ml−1 final concentration) for various durations, followed by medium changes. For repeated infections, the RV supernatant-containing medium was changed and maintained at 37 °C CO2 incubator for 1–2 h before adding fresh RV supernatant for next round of infection. At 24 h after infection, the cells were subjected to antibiotic selection.
Crystal violet staining
Crystal violet staining assay was conducted as described.33, 34, 35, 36 Briefly, at the end of antibiotic selection, the infected cells were washed with phosphate-buffered saline and stained with 0.5% crystal violet/formalin solution at room temperature for 20–30 min. The stained cells were washed with tap water and air-dried for taking macrographic images.37 For quantitative measurement, the stained cells were dissolved in 10% acetic acid at room temperature for 20 min with gentle shaking, followed by measuring absorbance at 570–590 nm.28, 38
Cloning and generation of recombinant adenoviral vectors AdPIT1 and AdPIT2
Recombinant adenoviruses were generated using the AdEasy technology as described.14, 15, 16 Briefly, the coding regions of human PIT1 and PIT2 were PCR-amplified and cloned into an adenoviral shuttle vector, and subsequently used to generate recombinant adenoviral vectors pAdPIT1 and pAdPIT2, which were used to generate recombinant adenoviruses in 293pTP cells. These recombinant adenoviruses also co-express enhanced green fluorescent protein. AdGFP was used as a control as previously described.39, 40, 41 Detailed information about vector constructs is available upon request.
RNA isolation and qPCR (touchdown qPCR)
Total RNA was isolated using TRIZOL Reagent (Invitrogen, Carlsbad, CA, USA) and subjected to reverse transcription with hexamer and M-MuLV Reverse Transcriptase (New England Biolabs, Ipswich, MA, USA). The cDNA products were diluted 10- to 100-fold and used as PCR templates. PCR primers were designed by using the Primer3 Plus program42 to human PIT1 and PIT2 (~120–250 bp; human PIT1: 5′-GTAGGGCCTGCCACTGTG-3′ and 5′-AAGTCCACGCTGGGAAGC-3′; human PIT2: 5′-CGATCGAGCTGGCCTCAG-3′ and 5′-ACGAACCAGGCCACGAAG-3′). The quantitative real-time-PCR analysis of the cDNA samples was carried out using our recently optimized touchdown qPCR protocol.43 Briefly, the SYBR Green qPCR reactions (Bio-Rad Laboratories, Hercules, CA, USA) were set up according to the manufacturer’s instructions. The cycling program was modified by incorporating four cycles of touchdown steps before the regular cycling program. GAPDH was used as a reference gene.
Statistical analysis
All quantitative experiments were performed in triplicate and/or repeated three times. Data were expressed as mean±s.d. Statistical significances between groups were determined by one-way analysis of variance and the Student’s t-test. A value of P<0.05 was considered statistically significant.
References
Temin HM . Reverse transcription in the eukaryotic genome: retroviruses, pararetroviruses, retrotransposons, and retrotranscripts. Mol Biol Evol 1985; 2: 455–468.
Baltimore D . Retroviruses and retrotransposons: the role of reverse transcription in shaping the eukaryotic genome. Cell 1985; 40: 481–482.
Coffin JM . Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol 1979; 42: 1–26.
Varmus H . Retroviruses. Science 1988; 240: 1427–1435.
D’Souza V, Summers MF . How retroviruses select their genomes. Nat Rev Microbiol 2005; 3: 643–655.
Temin HM . Genetics of retroviruses. Ann N Y Acad Sci 1995; 758: 161–165.
Jouvenet N, Laine S, Pessel-Vivares L, Mougel M . Cell biology of retroviral RNA packaging. RNA Biol 2011; 8: 572–580.
Ferrer M, Henriet S, Chamontin C, Laine S, Mougel M . From cells to virus particles: quantitative methods to monitor RNA packaging. Viruses 2016; 8: 8.
Dornburg R, Temin HM . Retroviral vector system for the study of cDNA gene formation. Mol Cell Biol 1988; 8: 2328–2334.
Wang G, Sinn PL, McCray PB Jr . Development of retroviral vectors for gene transfer to airway epithelia. Curr Opin Mol Ther 2000; 2: 497–506.
Miller AD . Retroviral vectors: from cancer viruses to therapeutic tools. Hum Gene Ther 2014; 25: 989–994.
Leverett BD, Farrell KB, Eiden MV, Wilson CA . Entry of amphotropic murine leukemia virus is influenced by residues in the putative second extracellular domain of its receptor, Pit2. J Virol 1998; 72: 4956–4961.
Feldman SA, Farrell KB, Murthy RK, Russ JL, Eiden MV . Identification of an extracellular domain within the human PiT2 receptor that is required for amphotropic murine leukemia virus binding. J Virol 2004; 78: 595–602.
He T-C . Adenoviral vectors. Current Protocols in Human Genetics. John Wiley & Sons, Inc.: New York, NY, USA, 2004, pp 12.4.1–12.4.25.
He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B . A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 1998; 95: 2509–2514.
Luo J, Deng ZL, Luo X, Tang N, Song WX, Chen J et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc 2007; 2: 1236–1247.
Miller AD . Development and applications of retroviral vectors. In: Coffin JM, Hughes SH, Varmus HE (eds). Retroviruses. Cold Spring Harbor: New York, NY, USA, 1997, pp 437–473.
Miller AD . Cell-surface receptors for retroviruses and implications for gene transfer. Proc Natl Acad Sci USA 1996; 93: 11407–11413.
Overbaugh J, Miller AD, Eiden MV . Receptors and entry cofactors for retroviruses include single and multiple transmembrane-spanning proteins as well as newly described glycophosphatidylinositol-anchored and secreted proteins. Microbiol Mol Biol Rev 2001; 65: 371–389, table of contents.
Grabarczyk P, Wysocki PJ, Gryska K, Mackiewicz A . Expression of PiT1 and PiT2 retroviral receptors and transduction efficiency of tumor cells. Acta Biochim Pol 2002; 49: 333–339.
Ozturk F, Park PJ, Tellez J, Colletti E, Eiden MV, Almeida-Porada G et al. Expression levels of the PiT-2 receptor explain, in part, the gestational age-dependent alterations in transduction efficiency after in utero retroviral-mediated gene transfer. J Gene Med 2012; 14: 169–181.
Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK . Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA 1993; 90: 8033–8037.
Ory DS, Neugeboren BA, Mulligan RC . A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci USA 1996; 93: 11400–11406.
Delwart EL, Panganiban AT . Role of reticuloendotheliosis virus envelope glycoprotein in superinfection interference. J Virol 1989; 63: 273–280.
Garcia JV, Miller AD . Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 1991; 350: 508–511.
Wu N, Zhang H, Deng F, Li R, Zhang W, Chen X et al. Overexpression of Ad5 precursor terminal protein accelerates recombinant adenovirus packaging and amplification in HEK-293 packaging cells. Gene Ther 2014; 21: 629–637.
Huang E, Bi Y, Jiang W, Luo X, Yang K, Gao JL et al. Conditionally immortalized mouse embryonic fibroblasts retain proliferative activity without compromising multipotent differentiation potential. PLoS One 2012; 7: e32428.
Wang N, Zhang W, Cui J, Zhang H, Chen X, Li R et al. The piggyBac transposon-mediated expression of SV40 T antigen efficiently immortalizes mouse embryonic fibroblasts (MEFs). PLoS One 2014; 9: e97316.
Deng Y, Zhang J, Wang Z, Yan Z, Qiao M, Ye J et al. Antibiotic monensin synergizes with EGFR inhibitors and oxaliplatin to suppress the proliferation of human ovarian cancer cells. Sci Rep 2015; 5: 17523.
Li Y, Wagner ER, Yan Z, Wang Z, Luther G, Jiang W et al. The calcium-binding protein S100A6 accelerates human osteosarcoma growth by promoting cell proliferation and inhibiting osteogenic differentiation. Cell Physiol Biochem 2015; 37: 2375–2392.
Luo Q, Kang Q, Song WX, Luu HH, Luo X, An N et al. Selection and validation of optimal siRNA target sites for RNAi-mediated gene silencing. Gene 2007; 395: 160–169.
Wen S, Zhang H, Li Y, Wang N, Zhang W, Yang K et al. Characterization of constitutive promoters for piggyBac transposon-mediated stable transgene expression in mesenchymal stem cells (MSCs). PLoS One 2014; 9: e94397.
Luo X, Chen J, Song WX, Tang N, Luo J, Deng ZL et al. Osteogenic BMPs promote tumor growth of human osteosarcomas that harbor differentiation defects. Lab Invest 2008; 88: 1264–1277.
Su Y, Luo X, He BC, Wang Y, Chen L, Zuo GW et al. Establishment and characterization of a new highly metastatic human osteosarcoma cell line. Clin Exp Metastasis 2009; 26: 599–610.
Su Y, Wagner ER, Luo Q, Huang J, Chen L, He BC et al. Insulin-like growth factor binding protein 5 suppresses tumor growth and metastasis of human osteosarcoma. Oncogene 2011; 30: 3907–3917.
Wang N, Zhang H, Zhang BQ, Liu W, Zhang Z, Qiao M et al. Adenovirus-mediated efficient gene transfer into cultured three-dimensional organoids. PLoS One 2014; 9: e93608.
Lamplot JD, Liu B, Yin L, Zhang W, Wang Z, Luther G et al. Reversibly immortalized mouse articular chondrocytes acquire long-term proliferative capability while retaining chondrogenic phenotype. Cell Transplant 2015; 24: 1053–1066.
He BC, Gao JL, Zhang BQ, Luo Q, Shi Q, Kim SH et al. Tetrandrine inhibits Wnt/beta-catenin signaling and suppresses tumor growth of human colorectal cancer. Mol Pharmacol 2011; 79: 211–219.
Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am 2003 85-A 8: 1544–1552.
Kang Q, Song WX, Luo Q, Tang N, Luo J, Luo X et al. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev 2009; 18: 545–559.
Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT et al. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther 2004; 11: 1312–1320.
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M et al. Primer3—new capabilities and interfaces. Nucleic Acids Res 2012; 40: e115.
Zhang Q, Wang J, Deng F, Yan Z, Xia Y, Wang Z et al. TqPCR: a touchdown qPCR assay with significantly improved detection sensitivity and amplification efficiency of SYBR green qPCR. PLoS One 2015; 10: e0132666.
Acknowledgements
We thank Dr Darell D Bigner of Duke University Medical Center for the generous provision of Duke263 cell line. The reported work was supported in part by research grants from the National Institutes of Health (AT004418 and DE020140 to TCH and RRR), the Department of Defense (OR130096 to JMW), the Scoliosis Research Society (TCH and MJL), the 973 Program of the Ministry of Science and Technology (MOST) of China (#2011CB707906 to TCH) and the National Natural Science Foundation of China (#81371972 and #81572142 to WH). JL was a recipient of the Predoctoral Fellowship from the China Scholarship Council, the Graduate Research and Innovation Project from Chongqing Education Commission and the Outstanding Predoctorate Research Fellowship from Chongqing Medical University (#CYB15098). This project was also supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant Number UL1 TR000430. Funding sources were not involved in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Liao, J., Wei, Q., Fan, J. et al. Characterization of retroviral infectivity and superinfection resistance during retrovirus-mediated transduction of mammalian cells. Gene Ther 24, 333–341 (2017). https://doi.org/10.1038/gt.2017.24
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2017.24
This article is cited by
-
Modeling lung diseases using reversibly immortalized mouse pulmonary alveolar type 2 cells (imPAC2)
Cell & Bioscience (2022)
-
Syncytin 1 dependent horizontal transfer of marker genes from retrovirally transduced cells
Scientific Reports (2019)
-
BMP9-induced osteoblastic differentiation requires functional Notch signaling in mesenchymal stem cells
Laboratory Investigation (2019)
-
Monensin inhibits cell proliferation and tumor growth of chemo-resistant pancreatic cancer cells by targeting the EGFR signaling pathway
Scientific Reports (2018)