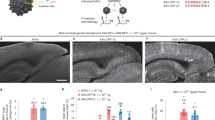
Abstract
Gene therapy for the central nervous system is poised to become a powerful treatment for numerous neurological disorders. Adeno-associated viral vectors based on serotype 9 (AAV9) have proven themselves to be strong candidates for delivering gene-based therapies throughout the brain and spinal cord when administered intravenously, intrathecally, intracisternally, and intracerebroventricularly (i.c.v.). Previous studies of i.c.v.-delivered self-complimentary AAV9 have been performed in neonatal mice with delivery of a single dose. However, before clinical trials can be considered, more information is required about the dose–response relationship for transduction efficiency in adult animals. In the current study, three doses of self-complementary AAV9 were administered to adult rats. High levels of transduction were observed in the hippocampus, cerebellum and cerebral cortex, and transduction increased with increasing dosage. Both neurons and astrocytes were transduced. There was no evidence of astrocytosis at the doses tested. Preliminary results from pigs receiving i.c.v. self-complementary AAV9 are also presented. The results of this study will serve to inform dosing studies in large animal models before clinical testing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gray SJ, Nagabhushan Kalburgi S, McCown TJ, Jude Samulski R . Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Therapy 2013; 20: 450–459.
Mingozzi F, High KA . Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood 2013; 122: 23–36.
Ellinwood NM, Ausseil J, Desmaris N, Bigou S, Liu S, Jens JK et al. Safe, efficient, and reproducible gene therapy of the brain in the dog models of Sanfilippo and Hurler syndromes. Mol Ther 2011; 19: 251–259.
Dayton RD, Wang DB, Klein RL . The advent of AAV9 expands applications for brain and spinal cord gene delivery. Expert Opin Biol Ther 2012; 12: 757–766.
McLean JR, Smith GA, Rocha EM, Hayes MA, Beagan JA, Hallett PJ et al. Widespread neuron-specific transgene expression in brain and spinal cord following synapsin promoter-driven AAV9 neonatal intracerebroventricular injection. Neurosci Lett 2014; 576: 73–78.
Levites Y, Jansen K, Smithson LA, Dakin R, Holloway VM, Das P et al. Intracranial adeno-associated virus-mediated delivery of anti-pan amyloid beta, amyloid beta40, and amyloid beta42 single-chain variable fragments attenuates plaque pathology in amyloid precursor protein mice. J Neurosci 2006; 26: 11923–11928.
Gholizadeh S, Tharmalingam S, Macaldaz ME, Hampson DR . Transduction of the central nervous system after intracerebroventricular injection of adeno-associated viral vectors in neonatal and juvenile mice. Hum Gene Ther Methods 2013; 24: 205–213.
Dirren E, Towne CL, Setola V, Redmond Jr DE, Schneider BL, Aebischer P . Intracerebroventricular injection of adeno-associated virus 6 and 9 vectors for cell type-specific transgene expression in the spinal cord. Hum Gene Ther 2014; 25: 109–120.
Chakrabarty P, Rosario A, Cruz P, Siemienski Z, Ceballos-Diaz C, Crosby K et al. Capsid serotype and timing of injection determines AAV transduction in the neonatal mice brain. PLoS One 2013; 8: e67680.
Broekman ML, Baek RC, Comer LA, Fernandez JL, Seyfried TN, Sena-Esteves M . Complete correction of enzymatic deficiency and neurochemistry in the GM1-gangliosidosis mouse brain by neonatal adeno-associated virus-mediated gene delivery. Mol Ther 2007; 15: 30–37.
Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011; 365: 2357–2365.
Haurigot V, Marco S, Ribera A, Garcia M, Ruzo A, Villacampa P et al. Whole body correction of mucopolysaccharidosis IIIA by intracerebrospinal fluid gene therapy. J Clin Invest 2013; 123: 3254–3271.
Bastianelli E . Distribution of calcium-binding proteins in the cerebellum. Cerebellum 2003; 2: 242–262.
Samaranch L, Salegio EA, San Sebastian W, Kells AP, Foust KD, Bringas JR et al. Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum Gene Ther 2012; 23: 382–389.
Sofroniew MV, Vinters HV . Astrocytes: biology and pathology. Acta Neuropathol 2010; 119: 7–35.
Benesova J, Hock M, Butenko O, Prajerova I, Anderova M, Chvatal A . Quantification of astrocyte volume changes during ischemia in situ reveals two populations of astrocytes in the cortex of GFAP/EGFP mice. J Neurosci Res 2009; 87: 96–111.
Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F et al. Uniquely hominid features of adult human astrocytes. J Neurosci 2009; 29: 3276–3287.
McCarty DM . Self-complementary AAV vectors; advances and applications. Mol Ther 2008; 16: 1648–1656.
McCarty DM, Monahan PE, Samulski RJ . Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Therapy 2001; 8: 1248–1254.
McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ . Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Therapy 2003; 10: 2112–2118.
Wang Z, Ma HI, Li J, Sun L, Zhang J, Xiao X . Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Therapy 2003; 10: 2105–2111.
Nathwani AC, Gray JT, Ng CY, Zhou J, Spence Y, Waddington SN et al. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood 2006; 107: 2653–2661.
Fontana A, Fierz W, Wekerle H . Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature 1984; 307: 273–276.
Samaranch L, San Sebastian W, Kells AP, Salegio EA, Heller G, Bringas JR et al. AAV9-mediated expression of a non-self protein in nonhuman primate central nervous system triggers widespread neuroinflammation driven by antigen-presenting cell transduction. Mol Ther 2014; 22: 329–337.
Hollak CE, Wijburg FA . Treatment of lysosomal storage disorders: successes and challenges. J Inherit Metab Dis 2014; 37: 587–598.
Sands MS, Davidson BL . Gene therapy for lysosomal storage diseases. Mol Ther 2006; 13: 839–849.
Xia H, Mao Q, Eliason SL, Harper SQ, Martins IH, Orr HT et al. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med 2004; 10: 816–820.
Costa Mdo C, Luna-Cancalon K, Fischer S, Ashraf NS, Ouyang M, Dharia RM et al. Toward RNAi therapy for the polyglutamine disease Machado-Joseph disease. Mol Ther 2013; 21: 1898–1908.
Acknowledgements
This work was funded through donations from the Virginia Gentlemen Foundation. The scAAV9-GFP for the rat studies was kindly provided by Marco Passini (Genzyme). Sectioning and staining of the pig brain was provided by RegenX.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Donsante, A., McEachin, Z., Riley, J. et al. Intracerebroventricular delivery of self-complementary adeno-associated virus serotype 9 to the adult rat brain. Gene Ther 23, 401–407 (2016). https://doi.org/10.1038/gt.2016.6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2016.6
This article is cited by
-
Reversibility of motor dysfunction in the rat model of NGLY1 deficiency
Molecular Brain (2021)
-
Destination Brain: the Past, Present, and Future of Therapeutic Gene Delivery
Journal of Neuroimmune Pharmacology (2017)