Abstract
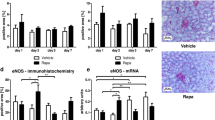
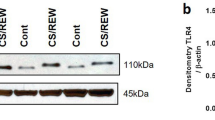
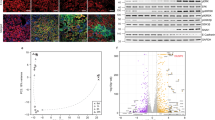
Chronic transplant dysfunction (CTD) is the primary cause of late allograft loss in kidney transplantation. Indoleamine 2,3-dioxygenase (IDO) is involved in fetomaternal tolerance and IDO gene therapy inhibits acute rejection following kidney transplantation. The aim of this study is to investigate whether gene therapy with IDO is able to attenuate CTD. Transplantation was performed in a rat Dark-Agouti to Wistar-Furth CTD model. Donor kidneys were incubated either with an adenovirus carrying IDO gene, a control adenovirus or saline. During the first 10 days recipients received low-dose cyclosporine. Body weight, blood pressure, serum creatinine and proteinuria were measured every 2 weeks. Rats were killed after 12 weeks. IDO had a striking beneficial effect on transplant vasculopathy at week 12. It also significantly improved body weight gain; it reduced blood pressure and decreased proteinuria during the follow-up. However, it did not affect the kidney function. In addition, IDO therapy significantly decreased the number of graft-infiltrating macrophages at week 12. The messenger RNA levels of forkhead box p3 and transforming grow factor-β were elevated in the IDO treated group at week 12. Here we show for first time a clear beneficial effect of local IDO gene therapy especially on transplant vasculopathy in a rat model of renal CTD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Savikko J, Rintala JM, Rintala SE, Koskinen PK, von WE . Early short-term imatinib treatment is sufficient to prevent the development of chronic allograft nephropathy. Nephrol Dial Transplant 2011; 26: 3026–3032.
Waanders F, Rienstra H, Boer MW, Zandvoort A, Rozing J, Navis G et al. Spironolactone ameliorates transplant vasculopathy in renal chronic transplant dysfunction in rats. Am J Physiol Renal Physiol 2009; 296: F1072–F1079.
Chapman JR, O'Connell PJ, Nankivell BJ . Chronic renal allograft dysfunction. J Am Soc Nephrol 2005; 16: 3015–3026.
Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE et al. Banff '05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy ('CAN'). Am J Transplant 2007; 7: 518–526.
Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T et al. The Banff 97 working classification of renal allograft pathology. Kidney Int 1999; 55: 713–723.
Halloran PF, Melk A, Barth C . Rethinking chronic allograft nephropathy: the concept of accelerated senescence. J Am Soc Nephrol 1999; 10: 167–181.
Paul LC . Chronic allograft nephropathy-a model of impaired repair from injury? Nephrol Dial Transplant 2000; 15: 149–151.
de Fijter JW . Rejection and function and chronic allograft dysfunction. Kidney Int Suppl 2010; 119: S38–S41.
Arend SM, Mallat MJ, Westendorp RJ, van der Woude FJ, van Es LA . Patient survival after renal transplantation; more than 25 years follow-up. Nephrol Dial Transplant 1997; 12: 1672–1679.
Joosten SA, Sijpkens YW, van KC, Paul LC . Chronic renal allograft rejection: pathophysiologic considerations. Kidney Int 2005; 68: 1–13.
Brandacher G, Cakar F, Winkler C, Schneeberger S, Obrist P, Bosmuller C et al. Non-invasive monitoring of kidney allograft rejection through IDO metabolism evaluation. Kidney Int 2007; 71: 60–67.
Mellor AL, Munn DH . IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 2004; 4: 762–774.
Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998; 281: 1191–1193.
Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 2003; 9: 1269–1274.
King NJ, Thomas SR . Molecules in focus: indoleamine 2,3-dioxygenase. Int J Biochem Cell Biol 2007; 39: 2167–2172.
Huang L, Baban B, Johnson BA III, Mellor AL . Dendritic cells, indoleamine 2,3 dioxygenase and acquired immune privilege. Int Rev Immunol 2010; 29: 133–155.
Li Y, Tredget EE, Ghaffari A, Lin X, Kilani RT, Ghahary A . Local expression of indoleamine 2,3-dioxygenase protects engraftment of xenogeneic skin substitute. J Invest Dermatol 2006; 126: 128–136.
Swanson KA, Zheng Y, Heidler KM, Mizobuchi T, Wilkes DS . CDllc+ cells modulate pulmonary immune responses by production of indoleamine 2,3-dioxygenase. Am J Respir Cell Mol Biol 2004; 30: 311–318.
Li J, Meinhardt A, Roehrich ME, Golshayan D, Dudler J, Pagnotta M et al. Indoleamine 2,3-dioxygenase gene transfer prolongs cardiac allograft survival. Am J Physiol Heart Circ Physiol 2007; 293: H3415–H3423.
Alexander AM, Crawford M, Bertera S, Rudert WA, Takikawa O, Robbins PD et al. Indoleamine 2,3-dioxygenase expression in transplanted NOD Islets prolongs graft survival after adoptive transfer of diabetogenic splenocytes. Diabetes 2002; 51: 356–365.
Ebrahimi A, Kardar GA, Toolabi L, Ghanbari H, Sadroddiny E . Inducible expression of indoleamine 2,3-dioxygenase attenuates acute rejection of tissue-engineered lung allografts in rats. Gene 2016; 576: 412–420.
Xiao B, Liu B, Song Y, Yu Z, Guo S . Local cytotoxic T-lymphocyte-associated antigen-4 immunoglobulin inhibition of rejection response is dependent on indoleamine 2,3-dioxygenase activities in the allograft. Transplant Proc 2014; 46: 3637–3640.
Vavrincova-Yaghi D, Deelman LE, Goor H, Seelen M, Kema IP, Smit-van OA et al. Gene therapy with adenovirus-delivered indoleamine 2,3-dioxygenase improves renal function and morphology following allogeneic kidney transplantation in rat. J Gene Med 2011; 13: 373–381.
Liu H, Liu L, Fletcher BS, Visner GA . Sleeping Beauty-based gene therapy with indoleamine 2,3-dioxygenase inhibits lung allograft fibrosis. FASEB J 2006; 20: 2384–2386.
Vavrincova-Yaghi D, Seelen MA, Kema IP, Deelman LE, van der Heuvel MC, Breukelman H et al. Early posttransplant tryptophan metabolism predicts long-term outcome of human kidney transplantation. Transplantation 2015; 99: e97–e104.
Sandovici M, Deelman LE, Smit-van OA, van GH, Rots MG, de ZD et al. Enhanced transduction of fibroblasts in transplanted kidney with an adenovirus having an RGD motif in the HI loop. Kidney Int 2006; 69: 45–52.
Kouwenhoven EA, Ijzermans JN, de Bruin RW . Etiology and pathophysiology of chronic transplant dysfunction. Transpl Int 2000; 13: 385–401.
Cook CH, Bickerstaff AA, Wang JJ, Nadasdy T, Della PP, Colvin RB et al. Spontaneous renal allograft acceptance associated with "regulatory" dendritic cells and IDO. J Immunol 2008; 180: 3103–3112.
Daley SR, Ma J, Adams E, Cobbold SP, Waldmann H . A key role for TGF-beta signaling to T cells in the long-term acceptance of allografts. J Immunol 2007; 179: 3648–3654.
Mitchell RN, Libby P . Vascular remodeling in transplant vasculopathy. Circ Res 2007; 100: 967–978.
Taflin C, Favier B, Baudhuin J, Savenay A, Hemon P, Bensussan A et al. Human endothelial cells generate Th17 and regulatory T cells under inflammatory conditions. Proc Natl Acad Sci USA 2011; 108: 2891–2896.
Hofmann F . Ido brings down the pressure in systemic inflammation. Nat Med 2010; 16: 265–267.
Wang Y, Liu H, McKenzie G, Witting PK, Stasch JP, Hahn M et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat Med 2010; 16: 279–285.
Rusai K, Schmaderer C, Hermans JJ, Lutz J, Heemann U, Baumann M . Direct renin inhibition in a rat model of chronic allograft injury. Transplantation 2011; 92: 999–1004.
Vavrinec P, van Dokkum RP, Goris M, Buikema H, Henning RH . Losartan protects mesenteric arteries from ROS-associated decrease in myogenic constriction following 5/6 nephrectomy. J Renin Angiotensin Aldosterone Syst 2011; 12: 184–194.
Jalili RB, Forouzandeh F, Rezakhanlou AM, Hartwell R, Medina A, Warnock GL et al. Local expression of indoleamine 2,3 dioxygenase in syngeneic fibroblasts significantly prolongs survival of an engineered three-dimensional islet allograft. Diabetes 2010; 59: 2219–2227.
Bickerstaff AA, Xia D, Pelletier RP, Orosz CG . Mechanisms of graft acceptance: evidence that plasminogen activator controls donor-reactive delayed-type hypersensitivity responses in cardiac allograft acceptor mice. J Immunol 2000; 164: 5132–5139.
Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF . Cutting edge: TGF-beta induces a regulatory phenotype in CD4+. J Immunol 2004; 172: 5149–5153.
Graca L, Thompson S, Lin CY, Adams E, Cobbold SP, Waldmann H . Both CD4(+)CD25(+) and CD4(+)CD25(-) regulatory cells mediate dominant transplantation tolerance. J Immunol 2002; 168: 5558–5565.
Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S et al. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol 2004; 172: 6003–6010.
Curti A, Pandolfi S, Valzasina B, Aluigi M, Isidori A, Ferri E et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of. Blood 2007; 109: 2871–2877.
Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol 2006; 176: 6752–6761.
Benigni A, Tomasoni S, Turka LA, Longaretti L, Zentilin L, Mister M et al. Adeno-associated virus-mediated CTLA4Ig gene transfer protects MHC-mismatched renal allografts from chronic rejection. J Am Soc Nephrol 2006; 17: 1665–1672.
Chen B, Kapturczak MH, Joseph R, George JF, Campbell-Thompson M, Wasserfall CH et al. Adeno-associated viral vector-mediated interleukin-10 prolongs allograft survival in a rat kidney transplantation model. Am J Transplant 2007; 7: 1112–1120.
Herrero-Fresneda I, Torras J, Franquesa M, Vidal A, Cruzado JM, Lloberas N et al. HGF gene therapy attenuates renal allograft scarring by preventing the profibrotic inflammatory-induced mechanisms. Kidney Int 2006; 70: 265–274.
Gong N, Dong C, Chen Z, Chen X, Guo H, Zeng Z et al. Adenovirus-mediated antisense-ERK2 gene therapy attenuates chronic allograft nephropathy. Transplant Proc 2006; 38: 3228–3230.
Yang J, Reutzel-Selke A, Steier C, Jurisch A, Tullius SG, Sawitzki B et al. Targeting of macrophage activity by adenovirus-mediated intragraft overexpression of TNFRp55-Ig, IL-12p40, and vIL-10 ameliorates adenovirus-mediated chronic graft injury, whereas stimulation of macrophages by overexpression of IFN-gamma accelerates chronic graft injury in a rat renal allograft model. J Am Soc Nephrol 2003; 14: 214–225.
Cassivi SD, Liu M, Boehler A, Pierre A, Tanswell AK, O'Brodovich H et al. Transplant immunosuppression increases and prolongs transgene expression following adenoviral-mediated transfection of rat lungs. J Heart Lung Transplant 2000; 19: 984–994.
Sandovici M, Deelman LE, van GH, Helfrich W, de ZD, Henning RH . Adenovirus-mediated interleukin-13 gene therapy attenuates acute kidney allograft injury. J Gene Med 2007; 9: 1024–1032.
Zaher SS, Coe D, Chai JG, Larkin DF, George AJ . Suppression of the allogeneic response by the anti-allergy drug N-(3,4-dimethoxycinnamonyl) anthranilic acid results from T-cell cycle arrest. Immunology 2013; 138: 157–164.
Sun QF, Ding JG, Sheng JF, Zhu MH, Li JJ, Sheng ZK et al. Novel action of 3,4-DAA ameliorating acute liver allograft injury. Cell Biochem Funct 2011; 29: 673–678.
Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol 1998; 72: 9706–9713.
He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B . A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 1998; 95: 2509–2514.
Reynolds P, Dmitriev I, Curiel D . Insertion of an RGD motif into the HI loop of adenovirus fiber protein alters the distribution of transgene expression of the systemically administered vector. Gene Ther 1999; 6: 1336–1339.
de Jong WH, Smit R, Bakker SJ, de Vries EG, Kema IP . Plasma tryptophan, kynurenine and 3-hydroxykynurenine measurement using automated on-line solid-phase extraction HPLC-tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 2009; 877: 603–609.
Acknowledgements
This work was supported by a Dutch Kidney Foundation grant, the Netherlands and by a Science Grant Agency (VEGA 1/0667/14 and VEGA 1/0223/15), Slovak Republic.
Author contributions
Vavrincova-Yaghi wrote the paper, participated in research design, performance of the research and data analyses. Deelman participated in revising the paper, research design and data analyses. van Goor participated in revising the paper, research design and immunohistology/histology analyses. Seelen participated in revising the paper, research design and data analyses. Vavrinec participated in performance of the research and data analyses. Kema participated in sample analyses and revising the paper. Gomolcak participated in immunohistology analysis. Benigni participated in revising the paper. Henning participated in revising the paper, research design and data analyses. Sandovici participated in revising the paper, research design and data analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Vavrincova-Yaghi, D., Deelman, L., van Goor, H. et al. Local gene therapy with indoleamine 2,3-dioxygenase protects against development of transplant vasculopathy in chronic kidney transplant dysfunction. Gene Ther 23, 797–806 (2016). https://doi.org/10.1038/gt.2016.59
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2016.59
This article is cited by
-
Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond
Nature Reviews Drug Discovery (2019)
-
Co-delivery of indoleamine 2,3-dioxygenase prevents loss of expression of an antigenic transgene in dystrophic mouse muscles
Gene Therapy (2017)