Abstract
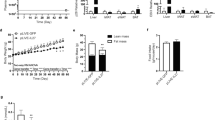
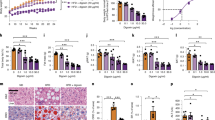
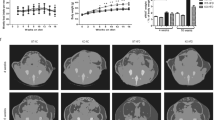
Obesity and its associated metabolic problems are a major public health issue. The objective of the current study is to investigate the therapeutic effects of interleukin 15/soluble interleukin 15 receptor-α (IL-15/sIL-15Rα) on high-fat diet-induced obesity and obesity-associated metabolic disorders. We demonstrate that the multiple hydrodynamic delivery of 2 μg IL-15/sIL-15Rα plasmid results in numerous beneficial effects, including a reduction of body weight and fat mass, an alleviation of fatty liver, an improvement in glucose homeostasis and insulin sensitivity in obese mice. These effects are accompanied by a suppressed expression of genes involved in lipid accumulation and lipogenesis, including Pparγ, Cd36, Fabp4, Mgat1, Scd-1 and Fas, and elevated mRNA levels of genes involved in adaptive thermogenesis and fatty acid β-oxidation, such as Ucp1, Ucp3, Pgc-1α, Pgc-1β, Pparα, Pparδ, Cpt1-α and Cpt1-β in obese animals. These results suggest that the overexpression of the Il-15/sIl-15Rα gene is an effective approach in treating diet-induced obesity and its associated metabolic complications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hill JO, Wyatt HR, Reed GW, Peters JC . Obesity and the environment: where do we go from here? Science 2003; 299: 853–855.
Ogden CL, Carroll MD, Kit BK, Flegal KM . Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 2014; 311: 806–814.
Egan B, Zierath JR . Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 2013; 17: 162–184.
Nieman DC, Davis JM, Henson DA, Walberg-Rankin J, Shute M, Dumke CL et al. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J Appl Physiol (1985) 2003; 94: 1917–1925.
Pedersen BK, Febbraio MA . Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 2012; 8: 457–465.
Quinn LS, Anderson BG . Interleukin-15, IL-15 receptor-alpha, and obesity: concordance of laboratory animal and human genetic studies. J Obes 2011 doi:10.1155/2011/456347.
Ma Y, Gao M, Sun H, Liu D . Interleukin-6 gene transfer reverses body weight gain and fatty liver in obese mice. Biochim Biophys Acta 2015; 1852: 1001–1011.
Gao M, Ma Y, Cui R, Liu D . Hydrodynamic delivery of FGF21 gene alleviates obesity and fatty liver in mice fed a high-fat diet. J Controlled Release 2014; 185: 1–11.
Gao M, Zhang C, Ma Y, Bu L, Yan L, Liu D . Hydrodynamic delivery of mIL10 gene protects mice from high-fat diet-induced obesity and glucose intolerance. Mol Ther 2013; 21: 1852–1861.
Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V et al. Cloning of a T-cell growth-factor that interacts with the beta-chain of the interleukin-2 receptor. Science 1994; 264: 965–968.
Tagaya Y, Bamford RN, DeFilippis AP, Waldmann TA . IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity 1996; 4: 329–336.
Nielsen AR, Hojman P, Erikstrup C, Fischer CP, Plomgaard P, Mounier R et al. Association between interleukin-15 and obesity: interleukin-15 as a potential regulator of fat mass. J Clin Endocrinol Metab 2008; 93: 4486–4493.
Barra NG, Reid S, MacKenzie R, Werstuck G, Trigatti BL, Richards C et al. Interleukin-15 contributes to the regulation of murine adipose tissue and human adipocytes. Obesity (Silver Spring) 2010; 18: 1601–1607.
Quinn LS, Anderson BG, Strait-Bodey L, Stroud AM, Argiles JM . Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. Am J Physiol Endocrinol Metab 2009; 296: E191–E202.
Quinn LS, Strait-Bodey L, Anderson BG, Argiles JM, Havel PJ . Interleukin-15 stimulates adiponectin secretion by 3T3-L1 adipocytes: evidence for a skeletal muscle-to-fat signaling pathway. Cell Biol Int 2005; 29: 449–457.
Barra NG, Palanivel R, Denou E, Chew MV, Gillgrass A, Walker TD et al. Interleukin-15 modulates adipose tissue by altering mitochondrial mass and activity. PLoS One 2014; 9: e114799.
Ajuwon KM, Spurlock ME . Direct regulation of lipolysis by interleukin-15 in primary pig adipocytes. Am J Physiol Regul Integr Comp Physiol 2004; 287: R608–R611.
Bergamaschi C, Rosati M, Jalah R, Valentin A, Kulkarni V, Alicea C et al. Intracellular interaction of interleukin-15 with its receptor alpha during production leads to mutual stabilization and increased bioactivity. J Biol Chem 2008; 283: 4189–4199.
Pedersen BK, Steensberg A, Schjerling P . Muscle-derived interleukin-6: possible biological effects. J Physiol 2001; 536 (Pt 2): 329–337.
Waldmann TA, Tagaya Y . The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol 1999; 17: 19–49.
Budagian V, Bulanova E, Paus R, Bulfone-Paus S . IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev 2006; 17: 259–280.
Sun H, Liu D . Hydrodynamic delivery of interleukin 15 gene promotes resistance to high fat diet-induced obesity, fatty liver and improves glucose homeostasis. Gene Therapy 2015; 22: 341–347.
Barra NG, Chew MV, Holloway AC, Ashkar AA . Interleukin-15 treatment improves glucose homeostasis and insulin sensitivity in obese mice. Diabetes Obes Metab 2012; 14: 190–193.
Alvarez B, Carbo N, Lopez-Soriano J, Drivdahl RH, Busquets S, Lopez-Soriano FJ et al. Effects of interleukin-15 (IL-15) on adipose tissue mass in rodent obesity models: evidence for direct IL-15 action on adipose tissue. Biochim Biophys Acta 2002; 1570: 33–37.
Sumithran P, Proietto J . The defence of body weight: a physiological basis for weight regain after weight loss. Clin Sci 2013; 124: 231–241.
Barra NG, Chew MV, Reid S, Ashkar AA . Interleukin-15 treatment induces weight loss independent of lymphocytes. PLoS One 2012; 7: e39553.
Kohl HW . Duration and intensity of exercise in weight loss among overweight women. Clin J Sport Med 2009; 19: 151–152.
Jakicic JM, Marcus BH, Gallagher KI, Napolitano M, Lang W . Effect of exercise duration and intensity on weight loss in overweight, sedentary women - a randomized trial. JAMA 2003; 290: 1323–1330.
Chambliss HO . Exercise duration and intensity in a weight-loss program. Clin J Sport Med 2005; 15: 113–115.
MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Peters JC, Hill JO . Metabolic adjustments with the development, treatment, and recurrence of obesity in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 2004; 287: R288–R297.
Carbo N, Lopez-Soriano J, Costelli P, Alvarez B, Busquets S, Baccino FM et al. Interleukin-15 mediates reciprocal regulation of adipose and muscle mass: a potential role in body weight control. Biochim Biophys Acta 2001; 1526: 17–24.
Almendro V, Fuster G, Busquets S, Ametller E, Figueras M, Argiles JM et al. Effects of IL-15 on rat brown adipose tissue: uncoupling proteins and PPARs. Obesity (Silver Spring) 2008; 16: 285–289.
Lopez-Soriano J, Carbo N, Almendro V, Figueras M, Ribas V, Busquets S et al. Rat liver lipogenesis is modulated by interleukin-15. Int J Mol Med 2004; 13: 817–819.
Ferre P . The biology of peroxisome proliferator-activated receptors: relationship with lipid metabolism and insulin sensitivity. Diabetes 2004; 53 (Suppl 1): S43–S50.
Berger JP, Akiyama TE, Meinke PT . PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci 2005; 26: 244–251.
Grimaldi PA . Regulatory role of peroxisome proliferator-activated receptor delta (PPAR delta) in muscle metabolism: a new target for metabolic syndrome treatment? Biochimie 2005; 87: 5–8.
Yu S, Matsusue K, Kashireddy P, Cao WQ, Yeldandi V, Yeldandi AV et al. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J Biol Chem 2003; 278: 498–505.
Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR et al. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem 2003; 278: 34268–34276.
Matsusue K, Haluzik M, Lambert G, Yim SH, Gavrilova O, Ward JM et al. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest 2003; 111: 737–747.
Almendro V, Busquets S, Ametller E, Carbo N, Figueras M, Fuster G et al. Effects of interleukin-15 on lipid oxidation: disposal of an oral [(14)C]-triolein load. Biochim Biophys Acta 2006; 1761: 37–42.
Busquets S, Figueras M, Almendro V, Lopez-Soriano FJ, Argiles JM . Interleukin-15 increases glucose uptake in skeletal muscle. An antidiabetogenic effect of the cytokine. Biochim Biophys Acta 2006; 1760: 1613–1617.
et al. Efficient systemic expression of bioactive IL-15 in mice upon delivery of optimized DNA expression plasmids. DNA Cell Biol 2007; 26: 827–840.
Schneider R, Campbell M, Nasioulas G, Felber BK, Pavlakis GN . Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J Virol 1997; 71: 4892–4903.
Rosati M, von Gegerfelt A, Roth P, Alicea C, Valentin A, Robert-Guroff M et al. DNA vaccines expressing different forms of simian immunodeficiency virus antigens decrease viremia upon SIVmac251 challenge. J Virol 2005; 79: 8480–8492.
Acknowledgements
We thank Dr Barbara K Felber (NCI) for generously providing us with AG209 DP muIL-15sRα+IL-15 plasmid and Mrs Francisca Burnley for proofreading the manuscript. The study was supported in part by grants from NIH (RO1 EB007357 and RO1 HL098295).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Sun, H., Ma, Y., Gao, M. et al. IL-15/sIL-15Rα gene transfer induces weight loss and improves glucose homeostasis in obese mice. Gene Ther 23, 349–356 (2016). https://doi.org/10.1038/gt.2016.4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2016.4
This article is cited by
-
A validated analysis pipeline for mass spectrometry-based vitreous proteomics: new insights into proliferative diabetic retinopathy
Clinical Proteomics (2021)
-
IL-25 stimulates M2 macrophage polarization and thereby promotes mitochondrial respiratory capacity and lipolysis in adipose tissues against obesity
Cellular & Molecular Immunology (2018)